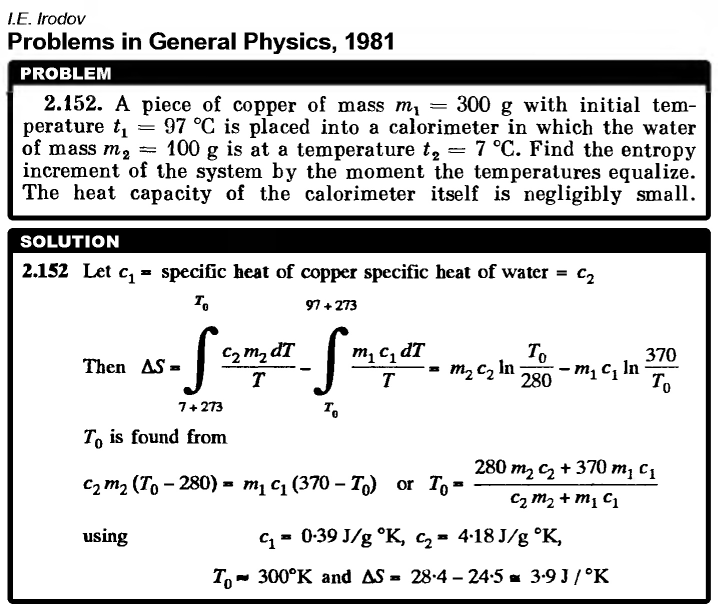
A piece of copper of mass m1 = 300 g with initial temperature t1 = 97 °C is placed into a calorimeter in which the water of mass m2 = 100 g is at a temperature t2 = 7 °C. Find the entropy increment of the system by the moment the temperatures equalize. The heat capacity of the calorimeter itself is negligibly small.  |
| New search. (Also 5349 free access solutions) |