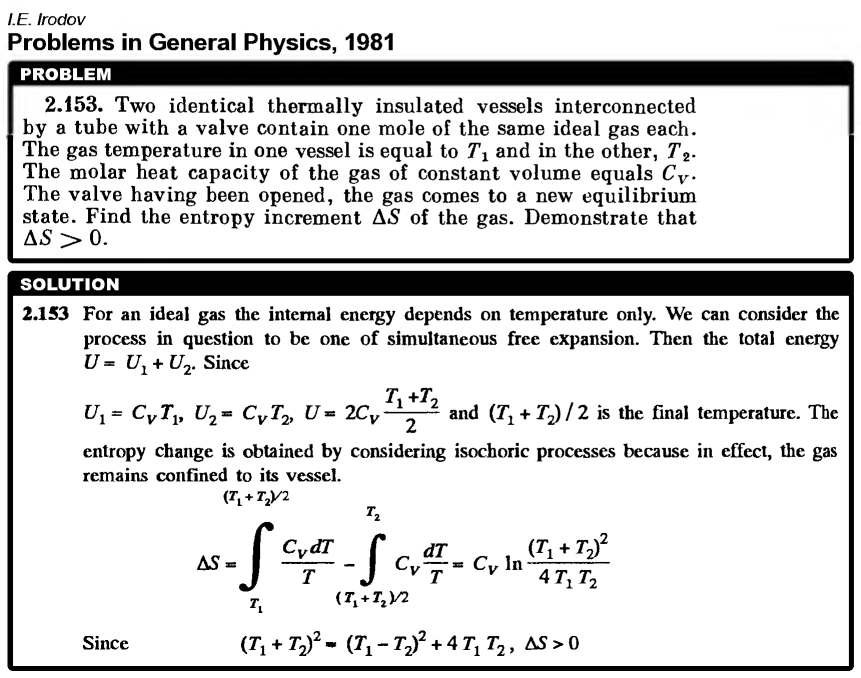
Two identical thermally insulated vessels interconnected by a tube with a valve contain one mole of the same ideal gas each. The gas temperature in one vessel is equal to T1 a nd in the other, T2. The molar heat capacity of the gas of constant volume equals Cv. The valve having been opened, the gas comes to a new equilibrium state. Find the entropy increment dS of the gas. Demonstrate that dS > 0. |
| New search. (Also 5349 free access solutions) |