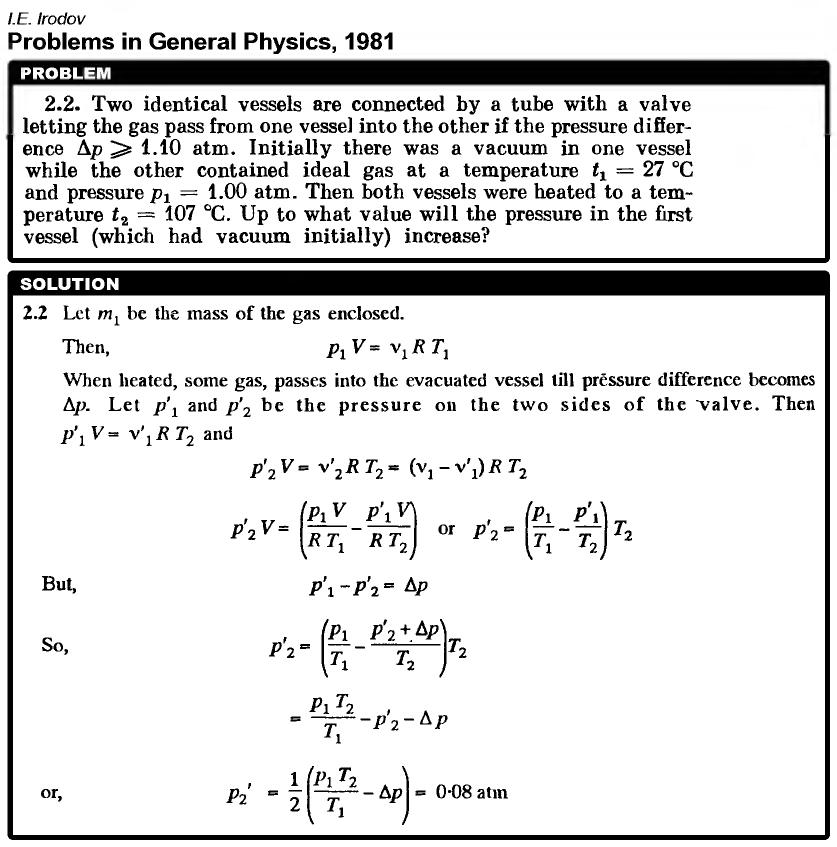
Two identical vessels are connected by a tube with a valve letting the gas pass from one vessel into the other if the pressure difference Δp ≥ 1.10 atm. Initially there was a vacuum in one vessel while the other contained ideal gas at a temperature t1 = 27 °C and pressure p1 = 1.00 atm. Then both vessels were heated to a temperature t2 = 107 °C. Up to what value will the pressure in the first vessel (which had vacuum initially) increase?  |
| New search. (Also 5349 free access solutions) |