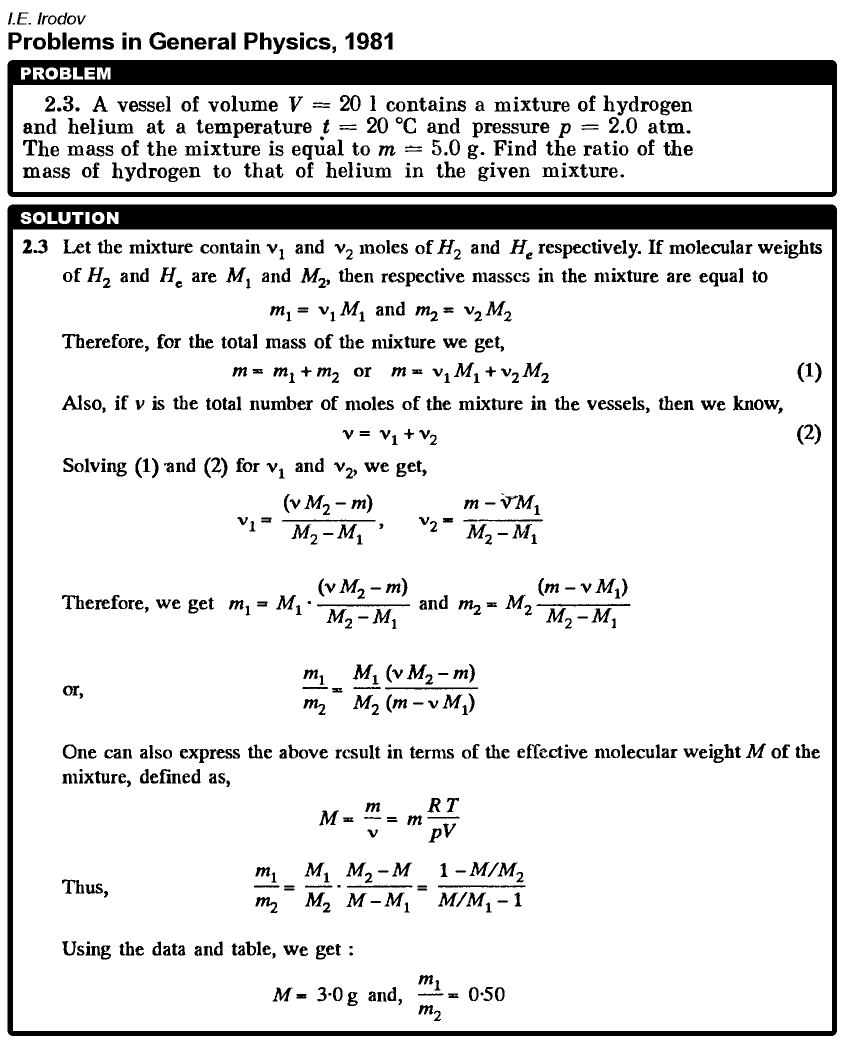
A vessel of volume V = 20 l contains a mixture of hydrogen and helium at a temperature t = 20 °C and pressure p = 2.0 atm. The mass of the mixture is equal to m = 5.0 g. Find the ratio of the mass of hydrogen to that of helium in the given mixture.  |
| New search. (Also 5349 free access solutions) |