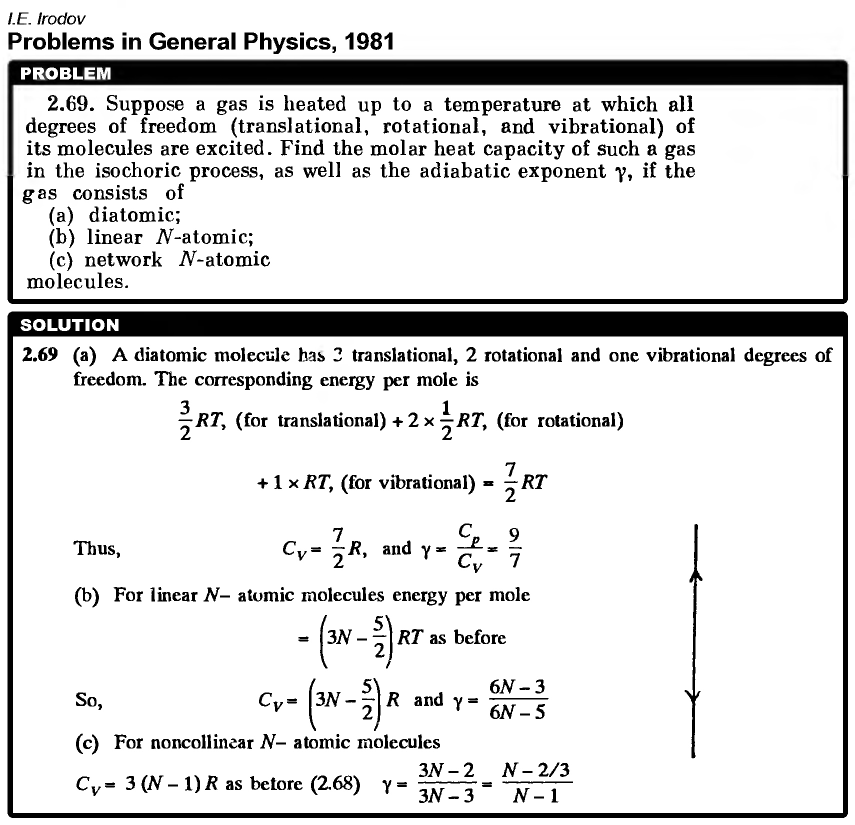
Suppose a gas is heated up to a temperature at which all degrees of freedom (translational, rotational, and vibrational) of its molecules are excited. Find the molar heat capacity of such a gas in the isochoric process, as well as the adiabatic exponent γ, if the gas consists of (a) diatomic; (b) linear N-atomic; (c) network N-atomic molecules.  |
| New search. (Also 5349 free access solutions) |