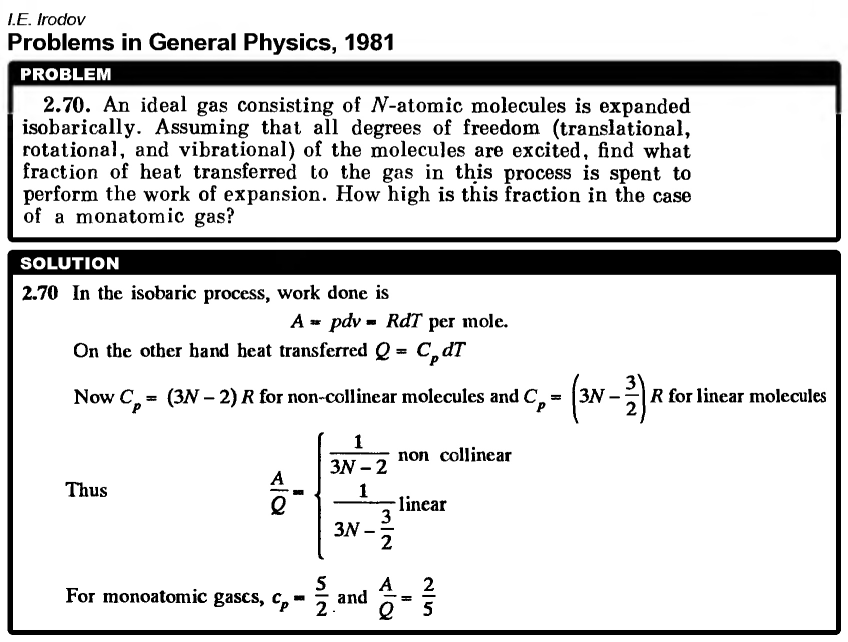
An ideal gas consisting of N-atomic molecules is expanded isobarically. Assuming that all degrees of freedom (translational, rotational, and vibrational) of the molecules are excited, find what fraction of heat transferred to the gas in this process is spent to perform the work of expansion. How high is this fraction in the case of a monatomic gas? |
| New search. (Also 5349 free access solutions) |