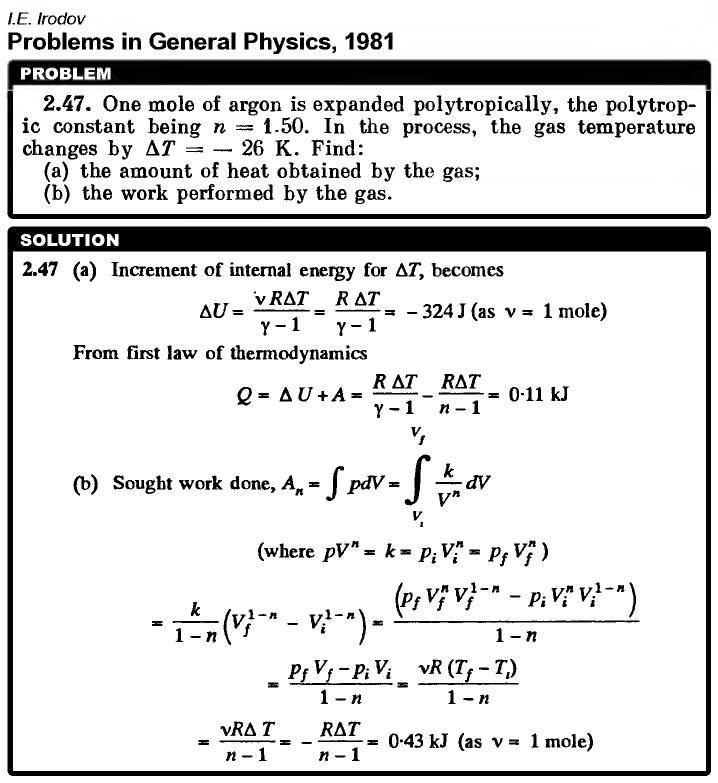
One mole of argon is expanded polytropically, the polytropic constant being n = 1.50. In the process, the gas temperature changes by ΔT = -26 K. Find: (a) the amount of heat obtained by the gas; (b) the work performed by the gas.  |
| New search. (Also 5349 free access solutions) |