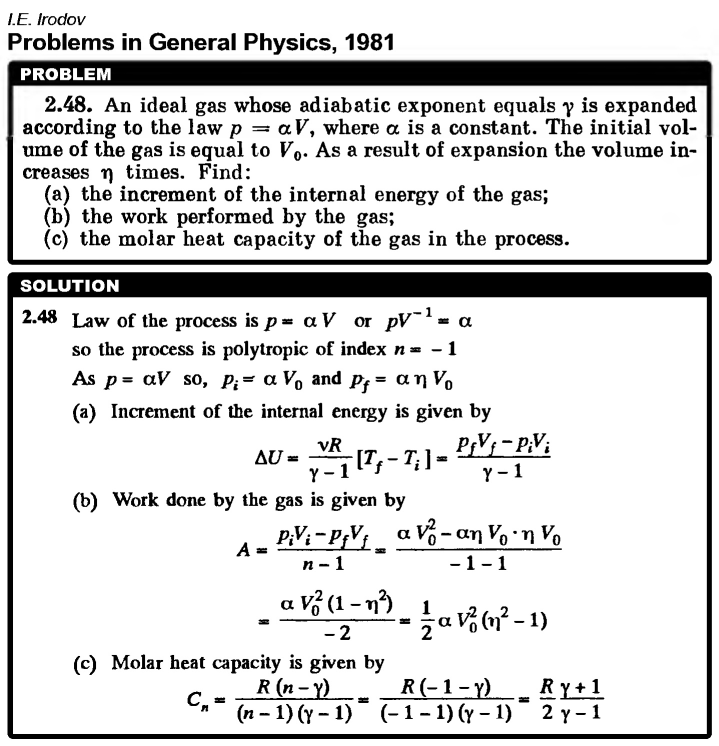
An ideal gas whose adiabatic exponent equals γ is expanded according to the law p = αV, where α is constant. The initial volume of the gas is equal to V0. As a result of expansion the volume increases η times. Find: (a) the increment of the internal energy of the gas; (b) the work performed by the gas; (c) the molar heat capacity of the gas in the process.  |
| New search. (Also 5349 free access solutions) |