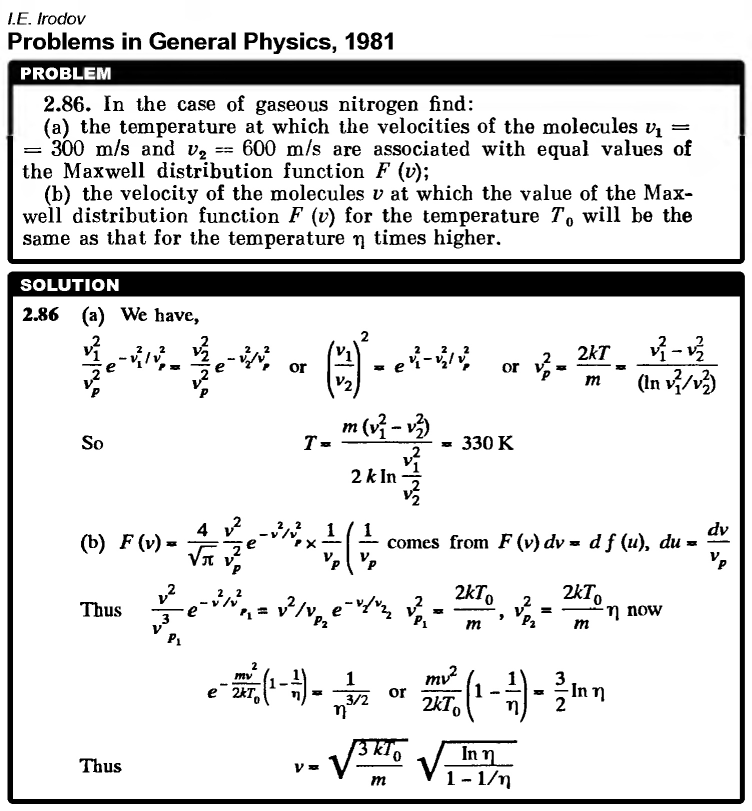
In the case of gaseous nitrogen find: (a) the temperature at which the velocities of the molecules v1 = 300 m/s and v2 = 600 m/s are associated with equal values of the Maxwell distribution function F(v); (b) the velocity of the molecules v at which the value of the Maxwell distribution function F(v) for the temperature T0 will be the same as that for the temperature η times higher.  |
| New search. (Also 5349 free access solutions) |