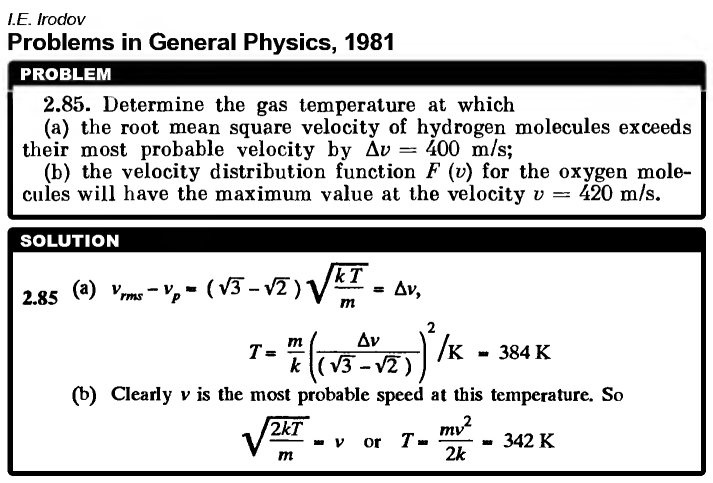
Determine the gas temperature at which (a) the root mean square velocity of hydrogen molecules exceeds their most probable velocity by Δv = 400 m/s; (b) the velocity distribution function F(v) for the oxygen molecules will have the maximum value at the velocity v = 420 m/s.  |
| New search. (Also 5349 free access solutions) |