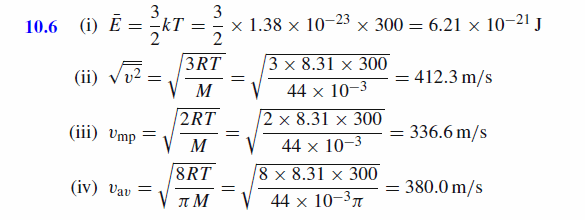
For carbon dioxide gas (CO2 , molar mass = 44.0 g/mol) at T = 300 K, calculate (i) the mean kinetic energy of one molecule (ii) the root mean square speed, vrms (iii) the most probable speed, vmp (iv) the average speed, vav (R = 8.31 J/K/mol, k = 1.38x10^(-23) J/K, NA = 6.02x10^(-23) /mol)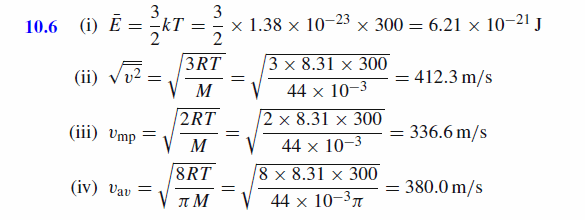 |
| New search. (Also 5349 free access solutions) |