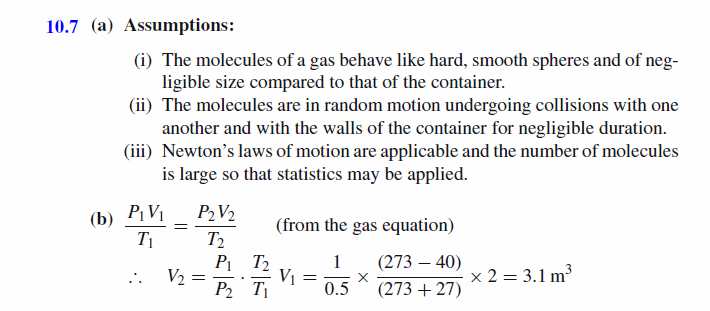
(a) Give three assumptions that are made when deriving the properties of an ideal gas using a molecular model. (b) A weather balloon is loosely filled with 2 m3 of helium at 1 atm. and 27°C. The balloon is then released, and by the time it has reached an elevation of 7000 m the pressure has dropped to 0.5 atm. and the balloon has expanded. If the temperature at this elevation is -48°C, what is the new volume of the balloon? |
| New search. (Also 5349 free access solutions) |