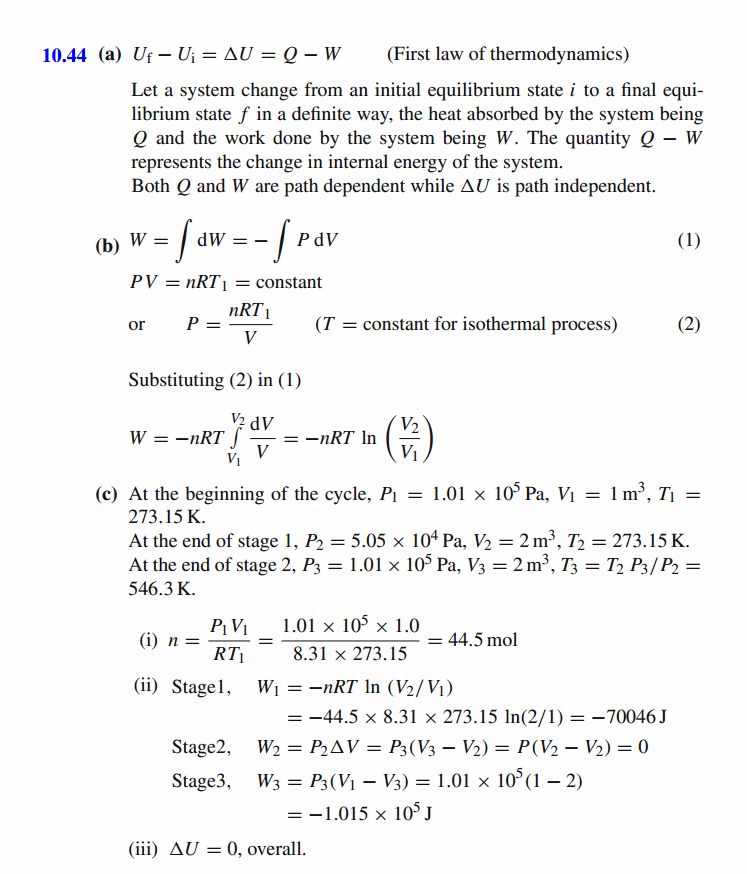
(a) State the first law of thermodynamics, expressing the law in its infinitesimal form. Explain carefully each term used and note whether or not each term is path dependent. (b) Show that the work done on a gas during an isothermal compression from an initial volume V1 to a final volume V2 is given by the equation W = -nRT ln (V2/V1) (c) An ideal gas system, with an initial volume of 1.0 m3 at standard temperature and pressure, undergoes the following three-stage cycle: Stage 1 - an isothermal expansion to twice its original volume. Stage 2 - a process by which its volume remains constant, its pressure returns to its original value and 104 J of heat is added to the system. Stage 3 - an isobaric compression to its original volume, with 3x10^4 J of heat being removed from the system. (i) How many moles of gas are present in the system? (ii) Calculate the work done on the system duri
Solution:
|

