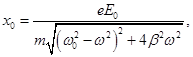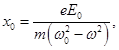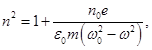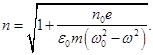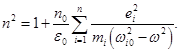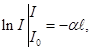main
To the list of lectures
|
Dispersion of light § 1 Method the observation of dispersion. Prismatic and diffraction spectra. Method Rozhdestvenskiy.
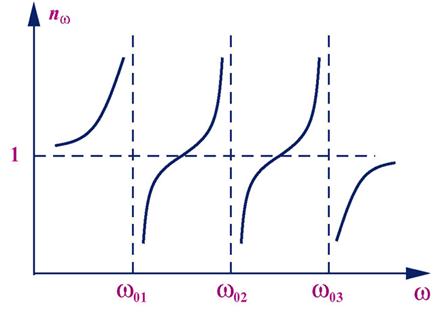
The phenomenon of dispersion is that the refractive index depends on the wavelength.
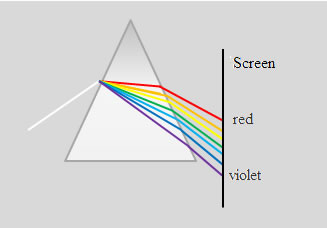
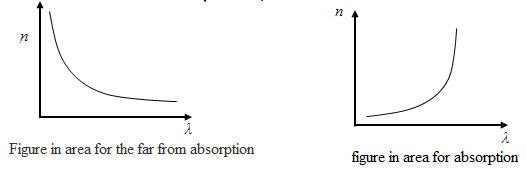
Spectrum shown in Fig. is prismatic. Violet waves are refracted more red, as wavelength red waves more than the purple, and the frequency, respectively, lower the refractive index of red waves will be smaller than the purple. Prism is often used in spectroscopy (spectroscopy (spectrometer, spectrograph) (from the range in the ancient Greek. Σκοπ?ω - see) - an optical instrument for visual observation of the radiation spectrum. Difference spectrometer of a spectrograph is that the spectrometer is a visual surveillance of the spectrum with through the eyes, and the spectrograph used a method of recording the spectrum - plate, recorder, photomultiplier or photocell, digital camera, etc. Both are used for the rapid qualitative spectral analysis of substances in chemistry, metallurgy (eg steeloscope) and m . g). Dispersion of light is called normal if the refractive index decreases monotonically with increasing wavelength (with increasing frequency).
In the case of dispersion if
dispersion of light is called anomalous. Normal dispersion of light seen in the distance from their own absorption lines, anomalous - within the lines or absorption lines.
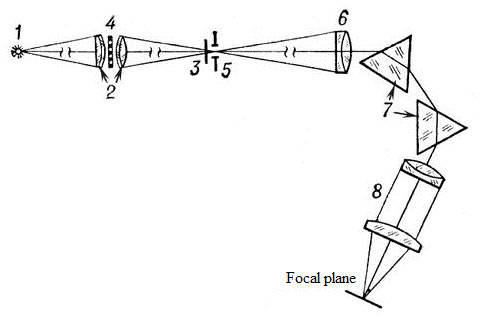
Optical scheme spektrosensitometra ISP-73: 1 - source of light (incandescent tungsten ribbon lamp), 2 - two-lens condenser 3 - disc shutter speeds of 0.05, 0.2 and 1.0 seconds, 4 - revolving disk with a set of perforated diaphragm; 5 - entrance slit of a spectrograph, 6 - collimator lens 7 - prism, 8 - the camera spectrograph. (Source: BSE).
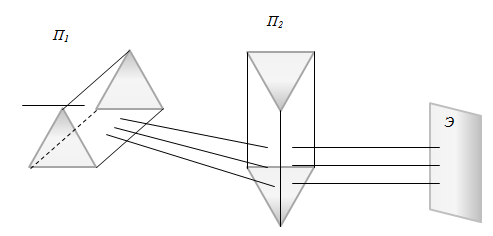
To study the normal and anomalous dispersion method can be used crossed prisms. Prism P1 - glass, P2 - from material dispersion which investigated. If the prism P2 would not have been on the screen E would be observed range of normal dispersion glass (Fig. 1). In the presence of the prism P2 is the curvature of the dispersion pattern at normal dispersion in P2, and the gap curved dispersion pattern - with anomalous dispersion. The method of
crossed prisms can not be used in the event that we are interested in n
gas emission, whose refractive index is close to 1. In this case,
D.S. Rozhdestvensky suggested that instead of a prism P1 put Jamin
interferometer, one arm of which is placed a sealed tube with gas, in
another - the plate, whose variance is known. Instead of a prism or
diffraction P2 put prismatic spectrograph (
§ 2 The electron theory of dispersion of light. Anomalous and normal dispersion of light. Relationship dispersion and absorption
For water, ε = 81, therefore ε(ω) < ε(0), поэтому n(ω) < n(0). ). Ie for each frequency will have its refractive index. Therefore, we must take into account the dependence of n on the frequency. The
phenomenon of dispersion can be explained by considering the
interaction of light waves with matter. This was made possible thanks
to the classical electron theory of Lorentz.
where χ - the dielectric susceptibility of the medium, P - polarization vector (the resultant dipole moment per unit volume). According to Maxwell's theory
In conditions when the substance of the incident light wave, the electric field is changing so rapidly that the polarizability (we need only electrons, ie, the induced field of the light wave) does not have time to change over the field. In this case
where n0 - the number of atoms per unit volume, PE - induced dipole moment of a single atom. It can be shown that the the highest exposures of the electric field of the light waves are more weakly associated with the core electrons, so-called optical electrons. For simplicity, we assume that each atom contains one optical electron. then
х - offset .
ie n depends on the displacement of electrons in the atom, the field of the light wave. On an electron in an atom acts as the forces: quasielastic - because of the presence of the electron with the nucleus:
resistance force:
The driving force of the light wave
Under action of these forces electron begins a forced oscillation
For simplicity we neglect the damping of oscillations. In this case,
If we consider the damping (β ≠ 0), then we get a formula which gives a good agreement with the experimental curve)
the Bouguer law
It was established experimentally that the light passes through a substance is absorbed. Particularly strong absorption was observed for the wavelengths, frequencies coincide with the natural frequencies for the substance. The intensity of light varies as:
where α - absorption coefficient, I0 - intensity of the incident light,
The thickness of the absorbing layer. The minus sign indicates that the dI and
the Bouguer law If
then
The absorption coefficient α is the value of the inverse value of the way in this matter, which is going through, the light decreases in intensity by a factor e. If the solution is light-absorbing material in a solvent which does not absorb the color, the absorption coefficient of the solution is directly proportional to the length of the absorbing material, ie
For sparse gas absorption spectrum is ruled. For the gas in the molecular state absorption spectrum is striped. For solid dielectric absorption spectrum of the solid in a certain range of frequencies. All other frequencies will pass the dielectric.
|