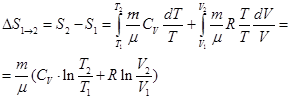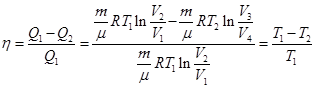main
To the list of lectures
|
§ 6 Entropy
Thermodynamic entropy and its meaning:
Entropy - is a function of the system, an infinitesimal change in a reversible process which is the ratio of the infinitesimal amount of heat introduced into the process, to the temperature at which it was introduced. All in a reversible process the entropy change can be calculated by the formula:
where the integration is one of the initial state 1 of the system to the final state 2. In any reversible process the change in entropy is equal to 0
2) In thermodynamics, it is proved that the system undergoes irreversible S cycle increases ΔS> 0 (2) Equations (1) and (2) apply only to closed systems, but if the system is exchanged heat with the environment, its S can behave in any way. Of (1) and (2) can be written as the Clausius inequality ΔS ≥ 0 ie entropy of a closed system can either increase (in the case of irreversible processes) or remain constant (in the case of reversible processes). If the system performs the equilibrium transition from state 1 to state 2, the entropy change
where dU and δA is recorded for a particular process. According to this formula ΔS is determined up to an additive constant. The physical meaning is not the entropy, entropy difference. We find the entropy change in the process of an ideal gas.
ie changes in the entropy S ΔS1 → 2 ideal gas at its transition from state 1 to state 2 is independent of the process. Because for an adiabatic process δQ = 0, ΔS = 0 => S = const, ie adiabatic reversible process takes place at constant entropy. So it is called isentropic. An isothermal process (T = const; T1 = T2:
When isochoric process (V = const; V1 =V2;
Entropy is additive: the entropy of the system is the sum of the entropies of bodies in the system. S = S1 + S2 + S3
+ ... Qualitative difference of the thermal motion of molecules from
other forms of movement is its state of chaos, randomness. Therefore,
to characterize the thermal motion to introduce a quantitative measure
of the degree of molecular disorder. If we consider any given
macroscopic state of the body with some average values, then it is
nothing but the continuous change of close microstates differing
distribution of molecules in different parts of the volume and the
energy is distributed between the molecules. These continuous
successive microstates characterizes the degree of disorder of the
macroscopic state of the entire system, ϖ is called thermodynamic
probability of the microstate. Thermodynamic probability ϖ of the
system - is the number of ways that this can be done state macroscopic
system, or the number of microstates carrying this microstate (ϖ ≥ 1,
and the mathematical probability of ≤ 1). According to Boltzmann, the entropy S of the system and the thermodynamic probability linked as follows:
where k - Boltzmann constant, Because real processes are irreversible, it can be argued that all the processes in a closed system leads to an increase in its entropy - the principle of entropy increase. In the statistical interpretation of entropy, this means that the process in a closed system are to increase the number of microstates, in other words, from less probable to more probable, as long as the probability of the state will not be maximized.
2) ΔS ≥ 0 (S = 0 for a reversible and ΔS ≥ 0 for an irreversible process) 3) According to Kelvin: circular process is not possible, the only result of which is the conversion of heat received from the heater into an equivalent work. 4) In the Clausius: circular process is not possible, the only result is to transfer heat from the less heated body to a warmer. To describe the thermodynamic systems at 0 K using Theorem Nernst-Planck (third thermodynamics): the entropy of all bodies in equilibrium tends to zero as the temperature approaches 0 K
From Theorem Nernst-Planck equation, it follows that Cp = Cv = 0 at 0 K.
Carnot cycle and its efficiency
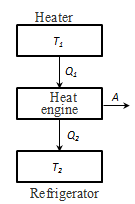
The principle of operation of the heat engine: the thermostat with temperature T1 - heater for a cycle is subtracted the amount of heat Q1, a thermostat with temperature T2 (T2 < T1), refrigerator, for a series of heat transferred to Q2, while work is done A = Q1 - Q2
Circular
process or cycle is a process by which the system is going through a
number of states, is reset. Cycle in the state diagram depicted a
closed curve. Cycle, performed by an ideal gas can be divided into
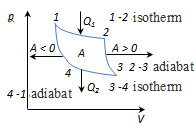
processes of expansion (1-2) and compression (2-1), the work of expansion is positive A1-2 > 0, because V2> V1, the work of compression is negative A1-2 < 0, because V2 < V1.
Hence, the work done by the gas per cycle, determined by the area
covered by a closed curve 1-2-1. If the cycle is done positive work
that is, the work done per cycle is the amount of heat received from the outside, but Q = Q1 - Q2 Q1 - the amount of heat received by the system Q2 - the amount of heat given system. Thermal efficiency for cyclic process is the ratio of the work done by the system, to the amount of heat supplied to the system:
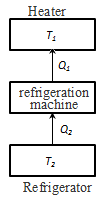
To η = 1, the condition Q2 = 0, ie, heat engine should have one heat source Q1, but this contradicts the second law of thermodynamics. The reverse process is happening in the heat engine is used in the refrigeration machine. The thermostat with temperature Т2 deducted the amount of heat Q2 transferred to the thermostat and the temperature T1, the amount of heat Q1. Without doing the work can not take away the heat from a hot body, and at least give it a warmer. Based on the second law of thermodynamics, Carnot led theorem.
1-2-isothermal expansion at T1 heater, gas is supplied to the heat Q1 and work is done
2 - 3 - adiabats. expansion, the gas does work A2-3 > 0 on external bodies. 3 - 4-isothermal compression at T2 refrigerator, heat is taken Q2 and work is done
4-1-adiabatic compression work is done on the gas A4-1 < 0 external bodies.
Adiabatic expansion Q2-3 = 0, and the work done A23 gas by the internal energy A23 = -U
The amount of heat Q2, uploaded from gas to refrigerator at isothermal compression equal to the work of compression А3-4
The work of the adiabatic compression
The work performed by a circular process
and equal to the area of ??the curve 1-2-3-4-1. Thermal efficiency Carnot cycle
From the equation for adiabatic processes 2-3 and 3-4 we obtain
then
ie efficiency Carnot cycle is determined only by the temperature heater and refrigerator. To increase the efficiency necessary to increase the difference Т1 - Т2.
|