main
To the list of lectures
|
§ 3 Bohr's postulates
By 1913, there were three experimental facts that can not be explained in terms of classical physics: 1) The empirical regularities of the line spectrum of the hydrogen atom, expressed in Balmer - Rydberg.
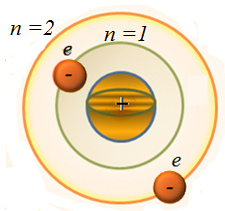
3) The quantum nature of the emission and absorption of light (heat radiation and the photoelectric effect). To be able to resolve the difficulty, Niels Bohr (Danish scientist) formulated three postulates for hydrogen and hydrogen-like atoms - a nucleus of charge Ze and a single electron moving around the nucleus. I - first postulate - the postulate of stationary states: In the system, there are some steady states that do not change over time without external influences. In these states, the atom emits no light. II – nd postulate - the rule of quantization of orbits: In the stationary state of the atom the electron moving in a circular orbit with the acceleration does not radiate light, must be discrete (quantized) values ??of angular momentum
III - rd postulate - the rule orbits:
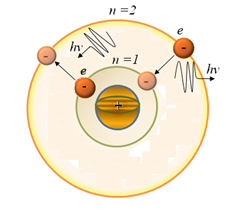
n > m – emission of a photon,
quantum transitions and determine the line spectrum of the atom.
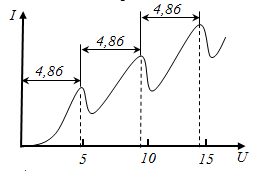
From experience, it follows that increasing the acceleration potential to 4.86 in the anode current increases monotonically. Passing at U = 4.86 ??V through maximum anode current drops sharply. Then increasing again when the U = 4.86 ??÷ 2 • 4.86 ??V. If U = 2 • 4.86 ??to falls and then rises again, etc. Closest to the ground state of the mercury atom is excited state, separated from the main 4.86 eV. As long as the potential difference UG1-K <4.86 electrons are in elastic collisions, and under the influence of the field fly to A. When UG1-K = 4.86 in the electron energy is absorbed by mercury vapor and the electron energy is not enough to overcome the retarding potential and etc. Mercury atoms to the ground state, emits light at λ = 255 nm (UV), which was found in the experiment. Thus, the experience of Franck and Hertz confirmed I and III - rd Bohr's postulates.
§ 5 The spectrum of the hydrogen atom in Bohr's model
By II - th postulate of Bohr
therefore,
The radius of the first Bohr orbit is r0= 0,529 Å r ~ n2. The internal energy of the atom is the sum of kinetic and potential energy
of
Follows
Then
Substitute in the expression for r, we obtain the allowed values ??of the energy:
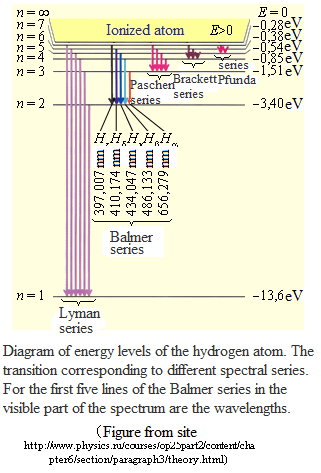
where the minus sign means that the electron is in a bound state. From (1) follows, that the energy states of the atoms form a sequence of energy levels, which vary depending on the value of n. Integer n in (1) determining the energy levels of the atom, called the principal quantum number. Energy state with n = 1 is the ground state. The state with n > 1 is called excited. The energy level to the ground state, called the ground, all others - excited.
Ionization of atoms - electron detachment from the atom. The ionization energy of the hydrogen atom is 13.6 eV.
According to II - th postulate of Bohr hydrogen atom in the transition from steady state n to the steady state m (n> m) emitted photon with energy
- Balmer - Rydberg formula, where
|