main
To the list of lectures
|
NUCLEAR PHYSICS Elements of quantum physics of atoms and molecules The doctrine of the atomic structure of matter originated in ancient times. However, before the end of XIX century atom considered a fundamental principle of indivisible unit ("brick") of any substance. In the
middle of the XIX century, it was experimentally shown that the
electron is one of the main components of any substance. (In 1749,
Benjamin Franklin hypothesized that electricity is a kind of material
substance. Central role of electrical matter, he assigned atomistic
conception of the structure of the electric fluid. In Franklin's work
first appeared terms: charge, discharge, positive charge, negative
charge, a capacitor, a battery particles of electricity. Johann Ritter in 1801 suggested discrete grain structure of electricity. In 1905, was proposed by JJ Thomson (Lord Kelvin), the first model of the atom, according to which the atom is continuously charged positively charged sphere of radius ~ 10-10 m, within which about their equilibrium positions fluctuate electrons. Net negative charge of the electrons is equal to the positive charge of the ball, so the atom as a whole is neutral (Thomson model of the atom is called "raisin bun" or "plum pudding").
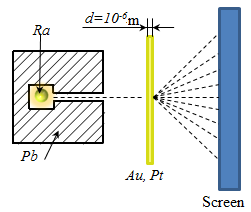
Rutherford's model of the atom Rutherford in 1909 conducted an experiment on the passage of α - particles through thin metal plates of gold and platinum. (α-particles are produced by radioactive transformations. charge α - particles equal to two charges of the electrons: qα = 2e = 2·1.6·10-19 Cl, the mass of the proton four masses: mα = 4 mp = 4·1.67·10 -27 kg). α - particles emitted by radium placed inside the cavity lead to the channel so that all particles except moving along the canal, absorbed lead. Narrow beam incident on a foil of gold, perpendicular to its surface. α - particles that have passed through the foil and scattered it in outbreaks (scintillations) on a fluorescent screen. Experiments have shown
that in most cases α - particles after passing through the foil, the
previous direction or rejected at very small angles. However, some α -
particles (about one in 20,000) were deflected at large angles, about
135 ÷ 150 °. Because electrons cannot significantly alter the
movement α - particles ( The
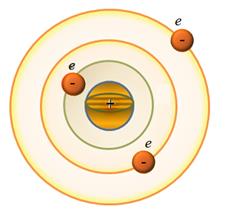
rest of the atom is a cloud of negatively charged electrons, the total
charge is equal to the positive charge of the nucleus. This model of a) The electrons in the atomic model can not be fixed, as the under the influence of the Coulomb force, they would be pulled (and "would fall") to the nucleus. In this model, there are infinitely many values ??of the radii of the orbits of the electron and the corresponding values ??of speed
This implies that the radius and velocity can change continuously. Consequently, it can emit any amount of energy, and therefore, the spectrum of the atom must be continuous. In fact, experience shows that atoms have a line spectrum. b) When r ≈10-10 m v≈ 106 m/s and
§ 2 The line spectrum of the hydrogen atom. Luminous gases produce a line emission spectra, consisting of individual spectral lines. When light passes through gases having absorption line spectra - each atom absorbs those spectral lines, which itself can emit. Spectrum - a set of harmonic components or wavelengths. For example, if the wave can be represented as a superposition of two waves with frequencies ω1 and ω2, then we say that the spectrum has two components or two lines with λ1 and λ2. The spectra are: a) ruled - from atoms and simple molecules discharged gases striped - complex molecules, solid - heated solids and liquids; b)
emission-in electric a gas discharge, heating of solids, etc.,
absorption - light passes through gases, liquids and solids, and thus
each atom absorbs those spectral lines, which itself can emit; First studied the spectrum of the simplest elements - hydrogen. Balmer in 1885 found that the wavelength known at the time the nine lines of the hydrogen spectrum can be calculated by the formula
J. Rydberg (Swedish scientist) offered some form of record
- Balmer - Rydberg formula. R’ = 10973731 m-1 – the Rydberg constant (R’ = 1.1·107 m-1), because
where R =R’c = 3.29·1015 s-1 – the same as the Rydberg constant. The Balmer – Rydberg formula first pointed to the special role of the integers in the spectral patterns.
All of the above series can be described by a single formula, called the generalized formula of Balmer
|
 .
.