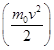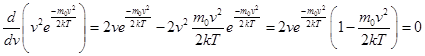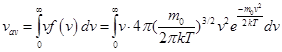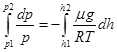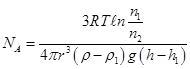main
To the list of lectures
|
§4 Maxwell's law of distribution of velocities and energies
To derive the function of the velocity distribution f(v) equal to the ratio of the number of molecules dN, the speed of which lie in the interval v ÷ v + dv to the total number of molecules N and the size of the interval dv
Maxwell used two sentences: a) all directions in space are equivalent and therefore any direction of the particle, ie, any direction of the velocity is equally likely. This property is sometimes called the property of the isotropic distribution function. b) moving along three mutually perpendicular axes that are independent, ie x-component of velocity vx does not depend on what the meaning of its components or vy, vz. And then the output f(v) is initially for one component vx, and then extended to all the coordinates of speed.
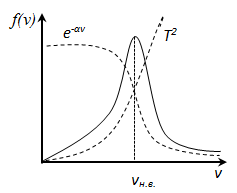
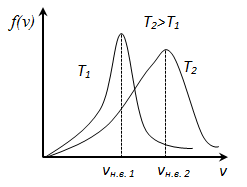
f (v) depends on the type of gas (the mass of the molecule) and the state variable (temperature T) For small v
In statistical physics, the average value of any quantity is defined as the integral from 0 to infinity, the product of the probability density for this value (statistical weight)
Then the arithmetic average velocity of the molecules
and integrating by parts received
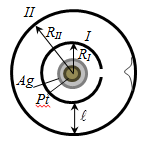
Speed, characterizing the state the gas § 5 Experimental verification of the Maxwell distribution law – Stern experience
Slit image
getting blurred. Exploring the thickness of the deposited layer can
estimate the distribution of the velocity, which corresponds to a
Maxwellian distribution.
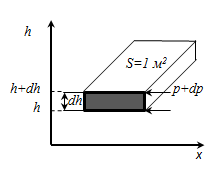
We derive the variation of pressure with height assuming that the gravitational field is uniform, the temperature is constant and the mass of all the molecules of the same. If the atmospheric pressure at a height h equal to p, then at a height h + dh is equal to p + dp (with dh> 0, dp <0, since p decreases with increasing h).
ρ - density at the height h, and since Then
Of Mendeleev-Clapeyron equation.
Then
With the change in height from h1 to h2 pressure changes from p1 to p2
Potentiated this expression
Barometric formula shows how the pressure changes with altitude At Then
Because the
and
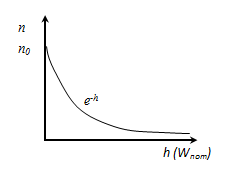
n - the concentration of molecules at a height n0 - the concentration of molecules at a height h = 0. Because the
the potential energy of the molecules in a gravitational field Boltzmann distribution law in an exterior potential field. From it follows that at T = const the density of gas is more there where potential energy of molecules is less.
§ 7 The experimental determination of the Avogadro constant J.
Perrin (French scientist) in 1909, studied the behavior of Brownian
particles in the emulsion gamboge (tree sap) with dimensions were
examined with a microscope, which had a depth of field - 1 mm. Moving
the microscope in the vertical direction could be to investigate the
distribution of Brownian particles in height.
where m- particle mass m1 - weight of the displaced fluid. If n1 and n2 concentration of particles at levels h1 and h2, and
then
Value which confirms the Boltzmann distribution of particles. |