|
§4 Radiant exitance Emissive power or irradiance. Stefan-Boltzmann law.
Wien's displacement law
RE
(integrated emissive power) - power emissive determines the amount of
energy emitted from the unit surface per unit time in the whole
frequency range from 0 to ∞ at a given temperature T.
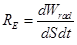
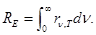
- relation irradiance and emittance.
[RE] =J/(m2·s) = W/m2
Law of J.Stephen (Austrian scientist) and L. Boltzmann (German scientist):
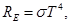
where
σ = 5.67·10-8 W/(m2· K4) - or Stefan- Boltzmann constant or Stefan's constant.
Emissive power of a black body is proportional to the fourth power of the thermodynamic temperature.
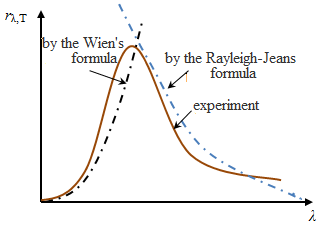
Stefan-Boltzmann law, determining the temperature dependence of the RE, is not the answer regarding the spectral composition of the radiation of a black body. From the experimental curves rλ,Т of λ at different T,
it follows that the spectral energy distribution of a black body is
uneven. All the curves have a maximum, which with increasing T is shifted to shorter wavelengths. The area bounded by the curve of rλ,Т of λ, is RE (this follows from the geometric meaning of the integral) and proportional to T4.
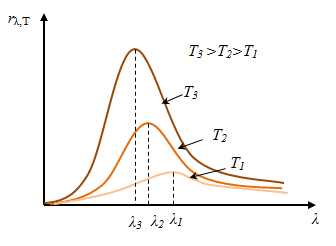 Wien's displacement law (1864 - 1928): The length of the waves (λmax),
which accounts for a maximum capacity of a black body emissivity at a
given temperature, is inversely proportional to the temperature T. Wien's displacement law (1864 - 1928): The length of the waves (λmax),
which accounts for a maximum capacity of a black body emissivity at a
given temperature, is inversely proportional to the temperature T.
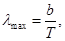
 b = 2,9· 10-3 m·K - Wien's displacement constant. b = 2,9· 10-3 m·K - Wien's displacement constant.
Wien's displacement is because as the temperature maximum emissivity is shifted to shorter wavelengths.
§5 Rayleigh-Jeans formula. Wien's law and ultraviolet catastrophe
Stefan-Boltzmann law allows to determine the energy emissive RE
black body on its temperature. Wien's displacement law binds the body
temperature with a wavelength, which accounts for the maximum
emissivity ability. But neither the one nor the other law does not
solve the basic problem of how high emissivity, the ability borne by
each λ in the spectrum of a black body at temperature T. For this we need to establish the functional dependence of rλ,Т of λ and T.
Based on the view of the continuous nature of the emission of
electromagnetic waves in the law of equipartition the degrees of freedom
was obtained two formulas for the black body emissivity ability:
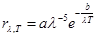
где а, b = const.
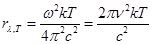
k = 1,38·10-23 J/K - Boltzmann constant
Experimental
analysis showed that for a given temperature of Wien is true for short
waves and gives sharp differences with experience in long waves. The
Rayleigh-Jeans was true for long waves and is not applicable for
short.
Study of the thermal
radiation with the aid of the Rayleigh-Jeans showed that in classical
physics can not resolve the question of the function characterizing the
emissivity of a black body This unsuccessful attempt to explain the laws
of black body radiation using the apparatus of classical physics is
called "ultraviolet catastrophe."
If you try to calculate the RE by the Rayleigh-Jeans formula, then
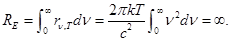
- "Ultraviolet catastrophe"
§6 Quantum hypothesis and Planck's formula
In 1900, Max Planck (German scientist) put forward the hypothesis
that the emission and absorption of energy is not continuous, but
certain small portions - quanta, the photon energy is proportional to
the frequency of the oscillations (Planck's formula):
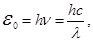
h = 6,625·10-34 J·s - Planck's constant or
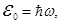
where 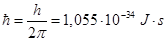
Since
the emission occurs in portions, the energy of the oscillator (the
vibrating atoms, electrons) E takes only the values ??integer multiples
of the elementary portions of energy, that is, only discrete values
Е = nЕо = n hν.
Photoelectric effect
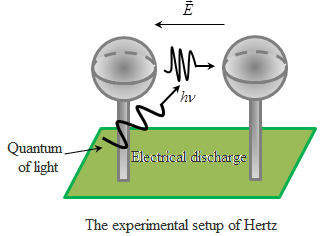 
The effect of the
light into the electrical processes were studied by Hertz in 1887. He
experimented with the electric discharge tube and found that the
irradiation of UV discharge occurs at a much lower voltage. In the
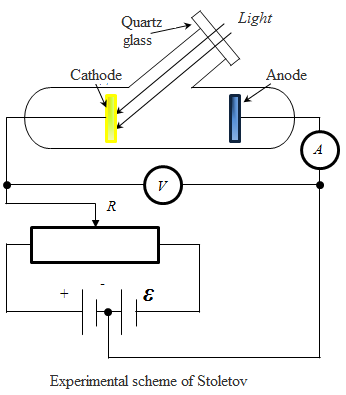
1889-1895 years. AG Stoletov studied the effects of light on metal,
using the following scheme. Two electrodes: the cathode to the study of
metal and anode A (scheme Stoletov - metal mesh, the transmitted
light) in a vacuum tube connected to the battery, so that by the
resistance R can change the value and sign of the voltage applied to
them. The irradiation of zinc cathode current is flowing in the
circuit, recorded milliammeter. Irradiating the cathode light of
different wavelengths, Stoletov established following pattern:
1. The most significant action has ultraviolet radiation;
2. By the light from the cathode break out the negative charges;
3. Current, due to the action of light, is directly proportional to its intensity.
Lenard and Thomson in 1898 measured the specific charge (e / m), pull
up the particles, and found that it is equal to the specific charge of
the electron, therefore, taken out of the cathode electrons.
§ 2 The external photoelectric effect. Three Laws of external photoeffect
External photoelectric effect is called electron emission substance
exposure to light. Electrons emitted from the material in the external
photoeffect, called photoelectrons and formed their current called
photocurrent.
With the scheme Stoletov obtained the following dependence of the
photocurrent on the applied voltage at a constant luminous flux Ф (that is, the CVC was obtained - the current-voltage characteristics):
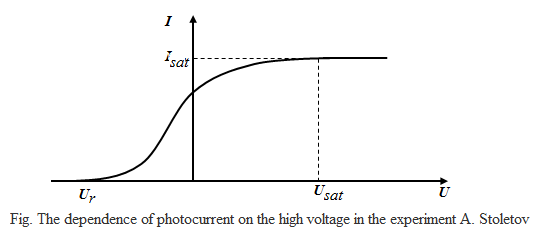
At a certain voltage Usat photocurrent reaches saturation Isat - all the electrons emitted by the cathode reach the anode, therefore, the current saturation Isat
determined by the number of electrons emitted by the cathode per unit
time under the action of light. The number of photoelectrons released
in proportion to the number of incident on the surface of the cathode
rays of light. And the number of photons is determined by the light
flux Ф, falling to the cathode. The number of photons N, falling for the time t on the surface is given by:
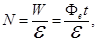
where W - the radiant energy received by the surface in time Δt,
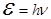 - The energy of the photon, - The energy of the photon,
Фе - luminous flux (radiant power).
1st law of the external photoeffect (law ??Stoletov):
At a fixed frequency of the incident light is proportional to the photocurrent saturation incident light flux:
Isat ~ Ф, ν = const
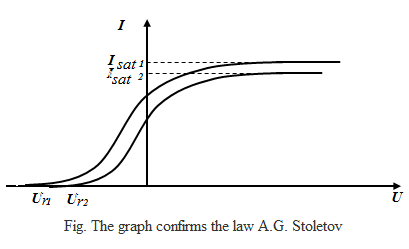
Ur
– retarding voltage - the voltage at which no single electron can not
reach the anode. Consequently, the law of conservation of energy, in
this case we can write the energy of the emitted electrons is delaying
the electric field energy
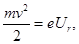
therefore, we can find the maximum speed of the emitted photoelectrons Vmax
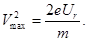
2 - second law photoelectric effect: maximum initial velocity Vmax photoelectrons does not depend on the intensity of the incident light (from Ф ), and is determined only its frequency ν
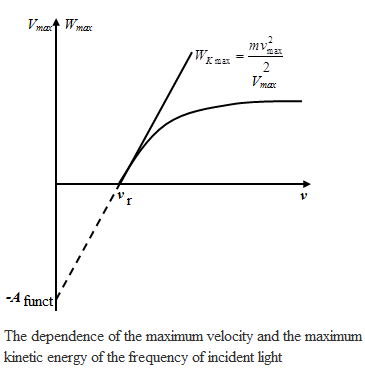 3rd
law of the photoelectric effect: for every matter there is a "red
edge'' photoelectric effect, that is, the minimum frequency νkp, depending on the chemical nature of matter and the state of its surface, which is still possible photoemission. 3rd
law of the photoelectric effect: for every matter there is a "red
edge'' photoelectric effect, that is, the minimum frequency νkp, depending on the chemical nature of matter and the state of its surface, which is still possible photoemission.
The second and third laws of the photoelectric effect can not be
explained by the wave nature of light (or the classical electromagnetic
theory of light). According to this theory tearing out conduction
electrons of a metal is a result of their "swinging" the
electromagnetic field of the light wave. With increasing light intensity
(Ф)
should increase the energy transferred to electrons in a metal,
therefore, be increased Vmax, which is contrary to the 2nd law of the
photoelectric effect.
Since by the wave theory of energy transfer is proportional to the intensity of the electromagnetic field intensity (Ф),
then any light, the frequency, but large enough intensity would pull
electrons from the metal, that is, a photoelectric threshold would not
exist, contrary to the 3rd law the photoelectric effect.
Photoemission is inertialess. A wave theory can not explain it without
inertia.
§3 The Einstein equation for the external photoelectric effect.
The work function
In 1905, Albert Einstein explained the photoelectric effect on the
basis of quantum concepts. According to Einstein, the light rays are
emitted not only in accordance with Planck's hypothesis, but is
distributed in space and is absorbed by the material separate portions -
rays with energy E0 = hv. Quanta of electromagnetic radiation called photons.
Einstein's equation (energy conservation law for the external photoelectric effect):
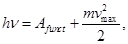
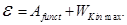
Incident photon energy hv that is spent on to work out electrons from the metal, and the message ejected photoelectron kinetic energy 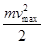 . .
Minimum energy that must be imparted to an electron in order to remove it from the solid into the vacuum is called the work function.
Since the Fermi energy EF to depend on temperature and EF, also changes with temperature, it follows that Asat depends on the temperature.
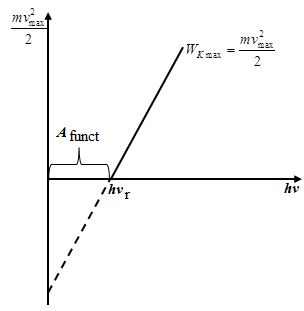 In addition, the work function is very sensitive to the surface finish. Causing the surface of the film (Ca, Sn, Ba) on W Asat decreases from 4.5 eV for pure W to 1.5 ¸ 2 eV for impurity W. In addition, the work function is very sensitive to the surface finish. Causing the surface of the film (Ca, Sn, Ba) on W Asat decreases from 4.5 eV for pure W to 1.5 ¸ 2 eV for impurity W.
Einstein's equation explains all thumbnails three laws external photoeffect
1st law: each quantum absorbed by only one electron. Therefore, the
number of photoelectrons taken out should be proportional to the
intensity of the (Ф) light
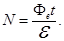
2nd law: Vmax ~ ν and because Asat is independent of Ф, then the Vmax is independent of Ф
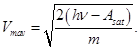
3rd Law: Reducing ν decreases Vmax and ν = ν0 Vmax= 0, therefore, hν0= Asat, therefore,  that there is a minimum frequency from which the possible photoemission. that there is a minimum frequency from which the possible photoemission.
|







