ELECTRICITY AND ELECTROMAGNETISM
ELECTROSTATICS. ELECTRIC FIELD IN VACUUM
§ 1 Atomistic charge. The elementary charge.
The law of conservation of charge
Under the charge to understand the physical properties of elementary
particles contaminated exerts a force on a charged particle Despite
the huge variety of substances in nature, there are only two types of
electric charges: positive, which arise, for example, the glass in
sliding his skin, and negative - in Ebony, worn on the fur.
Like charges repel, unlike charges - attract.

Electric charge
is discrete, ie charge of any body is an integer multiple of the
elementary electric charge: q = n e, where n-positive integer, e -
electron charge e = -1.6·10-19 C. Electron - an elementary carrier negative charge. Proton - nucleus of the hydrogen atom 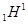 -
container carrier elements of positive charge. In the structure of the
hydrogen atom consists of one electron and one proton. Hydrogen atom,
as the atoms of other substances is neutral, that is, net plus charge of atom is equal to the net subzero charge Zp
= Ze. Atomistic charge is that the elementary negative and positive
charges are part of an atom in an isolated atom and they are always the
same number.
-
container carrier elements of positive charge. In the structure of the
hydrogen atom consists of one electron and one proton. Hydrogen atom,
as the atoms of other substances is neutral, that is, net plus charge of atom is equal to the net subzero charge Zp
= Ze. Atomistic charge is that the elementary negative and positive
charges are part of an atom in an isolated atom and they are always the
same number.
All bodies in nature can electrify, ie to get to
(give) an electric charge. The electrification of bodies can be done
in different ways, by contact (friction), electrostatic induction by
placing the body in an external electric field, etc. Every process
electrification reduced charge separation in which one of the bodies
(or body parts), there is an excess of positive charges, and the
another (or other body parts) - an excess of negative charges. The
total number of charges of both signs contained in the bodies does not
change, only the charges are redistributed between the bodies.
Electrically closed terms the system which is not exchanging charges with exterior bodies.
Charge conservation:
Algebraic
sum of the electrical charges of any closed system remains constant,
no matter what the process would not occur within the system
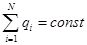
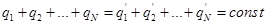
By
the ability to pass an electrical current (ie charge transfer), all
substances are divided into conductors, semiconductors and insulators.
CONDUCTORS
body in which an electric charge can be moved over the entire volume
of the conductor. Lead resistance is small, and the conductivity is
high.
The conductors are divided into two groups:
I
kind of conductors - metals - the charge carriers are electrons. Flow
of electrical charge does not lead to chemical changes of matter.
Conductors
II kind - solutions of acids, salts and melts - the charge carriers -
electrons and ions, they transfer charges leads to chemical changes
(electrolysis).
Dielectric (insulator) matter not conducting
electrical current (glass, air, plastic, etc.). There are no free
charges in the conductors, all charges related to the molecules of the
dielectric. High resistance, conductivity is low.
Semiconductors -
under certain conditions (high temperatures and electric fields) are
able to conduct electricity (germanium, silicon, gallium arsenide).
A unit of electrical charge - coulomb - l electric charge passing through a cross-section at a current of 1 amp for the time of 1 s.
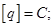
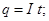
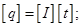
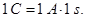
§ 2 Coulomb's law
Point charges are called charged bodies, the size of which can be neglected in comparison with the distance between them.
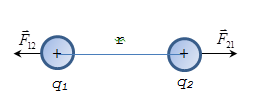
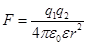
Coulomb's law: Two fixed point charges interact with the force F
is directly proportional to the magnitude of the charges and inversely
proportional to the square of the distance between them.
Coulomb force directed along the line joining the interacting charges, ie is central. F < 0 for opposite charges (charges attract); F > 0 for the same charges (charges repel).
Coulomb's law in vector form:
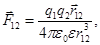
where 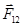 - the force on the 1st of the charge of the 2nd,
- the force on the 1st of the charge of the 2nd,
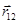 - radius-vector between charges 1 and 2.
- radius-vector between charges 1 and 2.
ε0 - electric constant; ε0 = 8.85 · 10-12 F / m,
F - farad - unit capacity;
ε -
dielectric constant of the medium, shows how many times the force
between two point charges in the medium is less than the force of
interaction in a vacuum, if the distance between the charges is not
changed
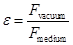
ε indicates weakening Coulomb force (and the electrostatic field) in the medium compared to vacuum.
[ε]=1.
According to Newton's third law