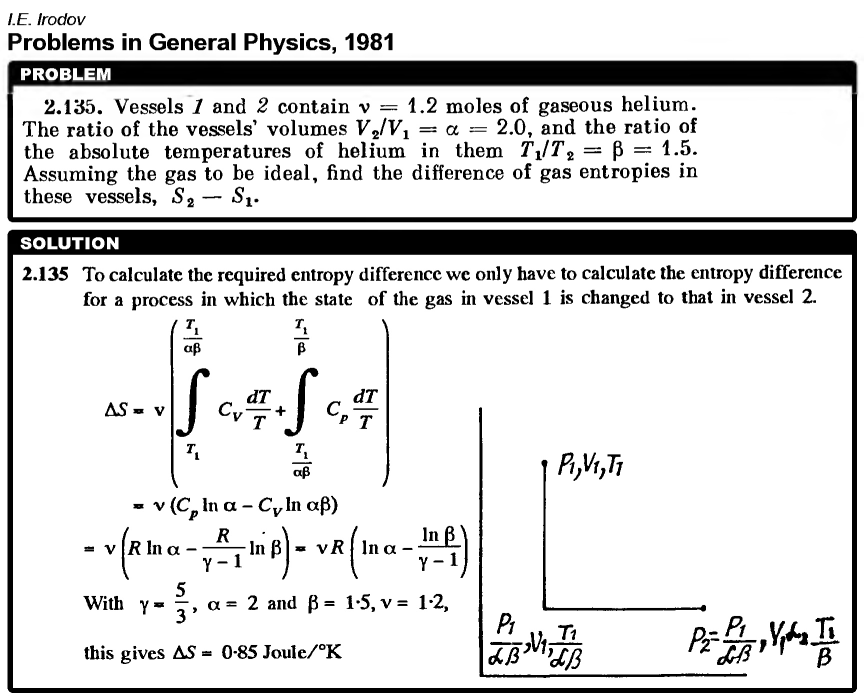
Vessels 1 and 2 contain Λ = 1.2 moles of gaseous helium. The ratio of the vessels' volumes V2/V1 = α = 2.0, and the ratio of the absolute temperatures of helium in them T1/T2 = β = 1.5. Assuming the gas to be ideal, find the difference of gas entropies in these vessels, S2 - S1.  |
| New search. (Also 5349 free access solutions) |