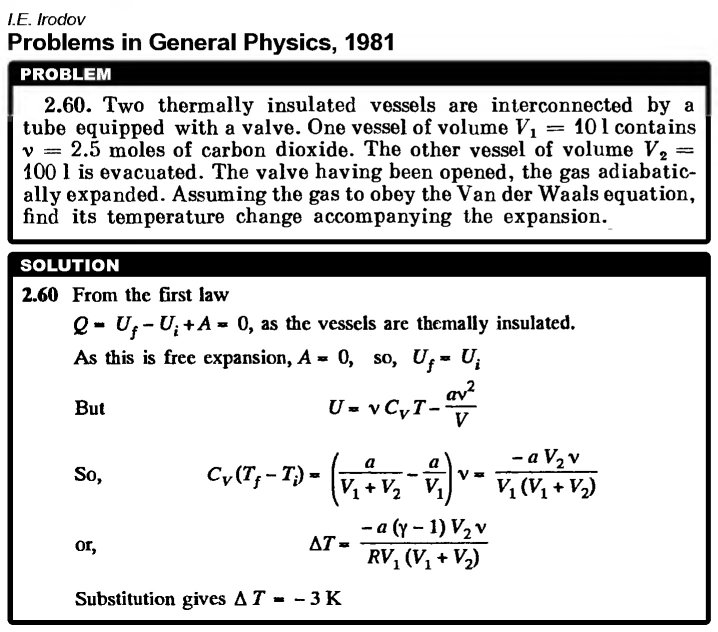
Two thermally insulated vessels are interconnected by a tube equipped with a valve. One vessel of volume V1 = 10 l contains ν = 2.5 moles of carbon dioxide. The other vessel of volume V2 = 100 l is evacuated. The valve having been opened, the gas adiabatically expanded. Assuming the gas to obey the Van der Waals equation, find its temperature change accompanying the expansion.  |
| New search. (Also 5349 free access solutions) |