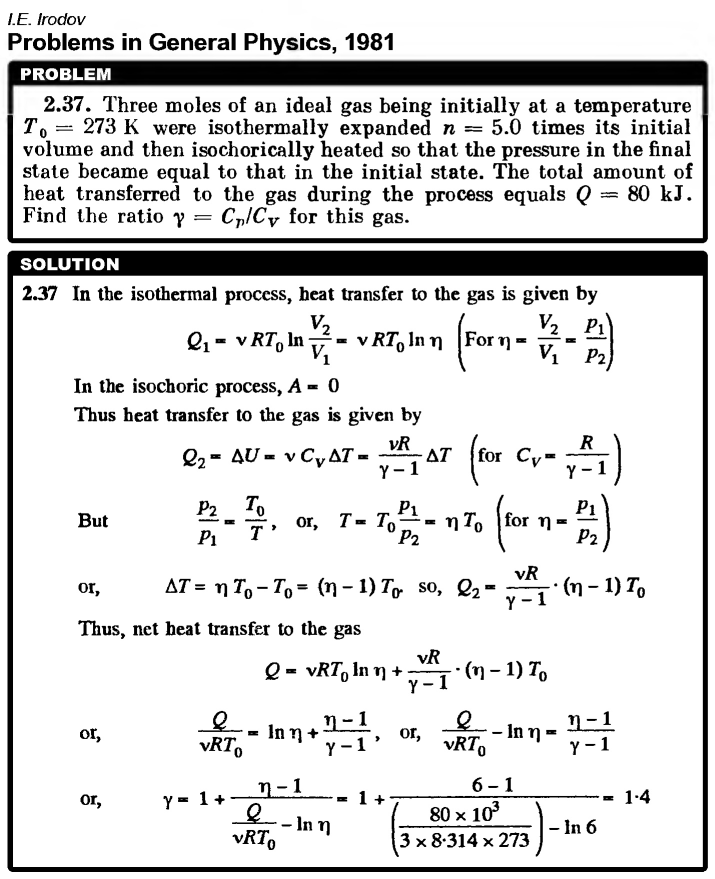
Three moles of an ideal gas being initially at a temperature T0 = 273 K were isothermally expanded n = 5.0 times its initial volume and then isochorically heated so that the pressure in the final state became equal to that in the initial state. The total amount of heat transferred to the gas during the process equals Q = 80 kJ. Find the ratio γ = Cp/CV for this gas.  |
| New search. (Also 5349 free access solutions) |