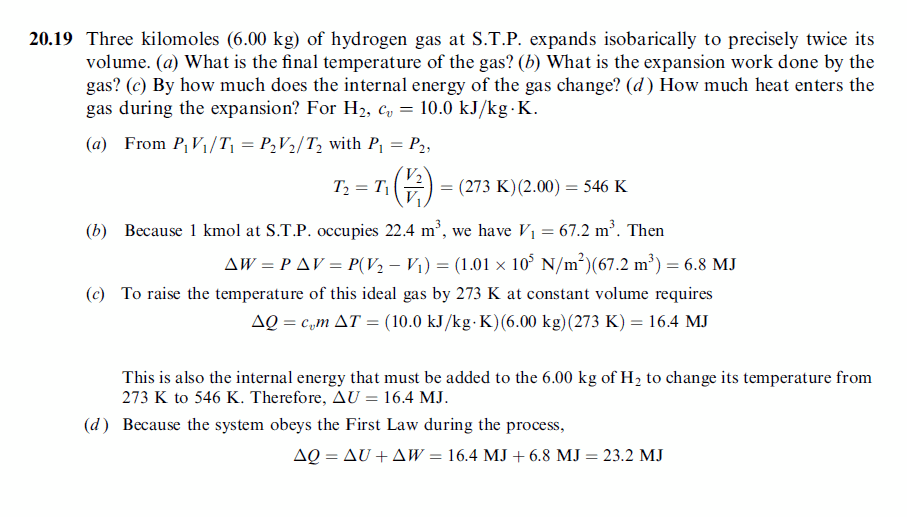
Three kilomoles (6.00 kg) of hydrogen gas at S.T.P. expands isobarically to precisely twice its volume. (a) What is the final temperature of the gas? (b) What is the expansion work done by the gas? (c) By how much does the internal energy of the gas change? (d) How much heat enters the gas during the expansion? For H2, cv = 10.0 kJ/kg·K.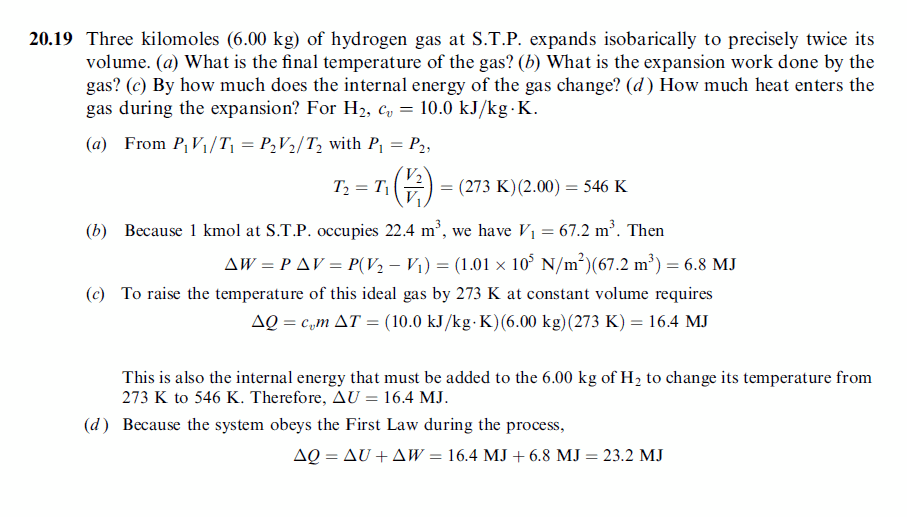 |
| New search. (Also 5349 free access solutions) |