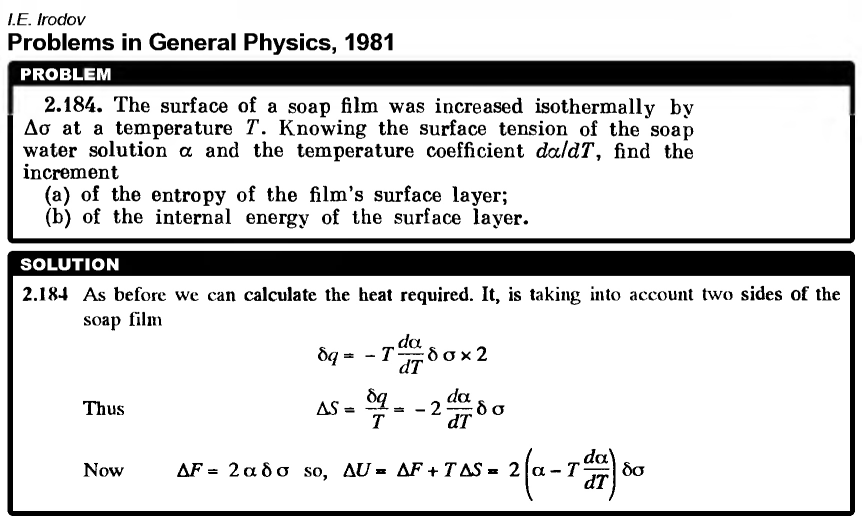
The surface of a soap film was increased isothermally by ds at a temperature T. Knowing the surface tension of the soap water solution a and the temperature coefficient da/dT, find the increment (a) of the entropy of the film's surface layer; (b) of the internal energy of the surface layer. |
| New search. (Also 5349 free access solutions) |