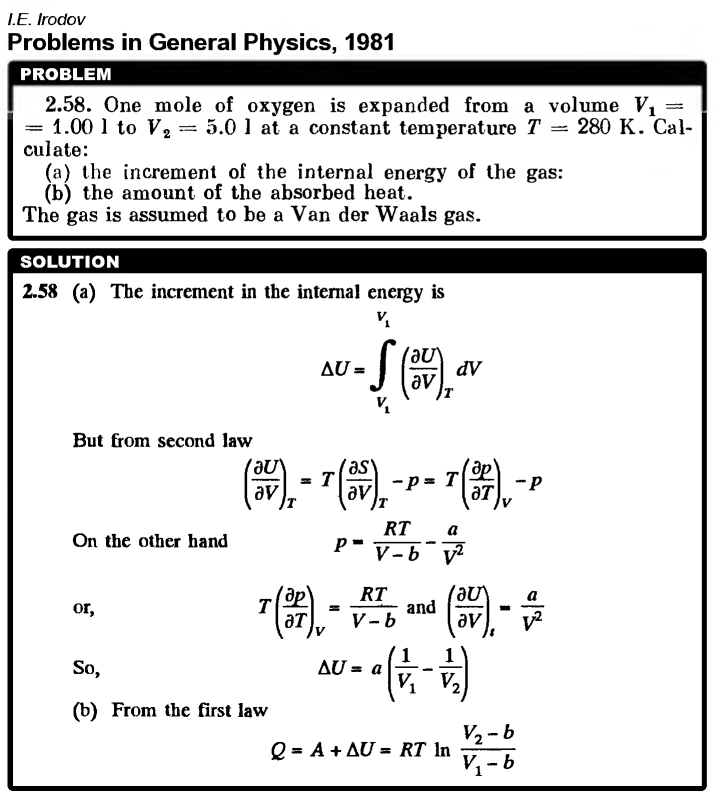
One mole of oxygen is expanded from a volume V1 = 1.00 L to V2 = 5.0 L at a constant temperature T = 280 K. Calculate: (a) the increment of the internal energy of the gas: (b) the amount of the absorbed heat. The gas is assumed to be a Van der Waals gas. |
| New search. (Also 5349 free access solutions) |