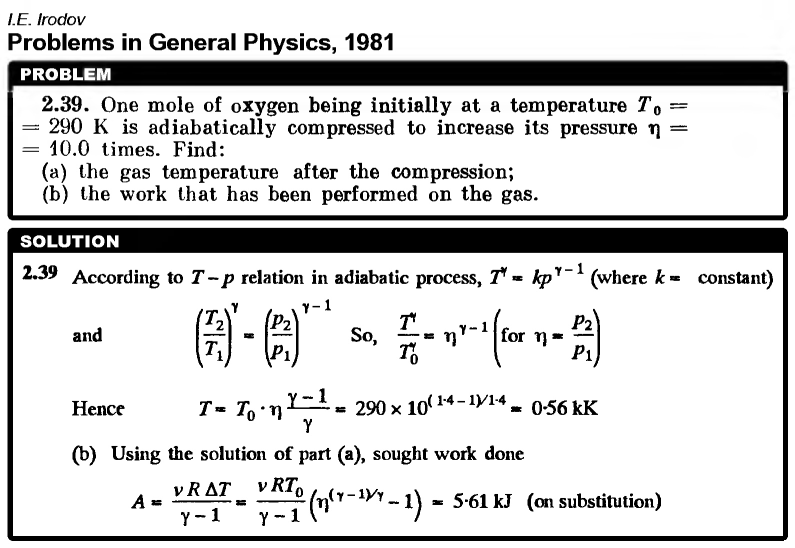
One mole of oxygen being initially at a temperature T0 = 290 K is adiabatically compressed to increase its pressure η = 10.0 times. Find: (a) the gas temperature after the compression; (b) the work that has been performed on the gas.  |
| New search. (Also 5349 free access solutions) |