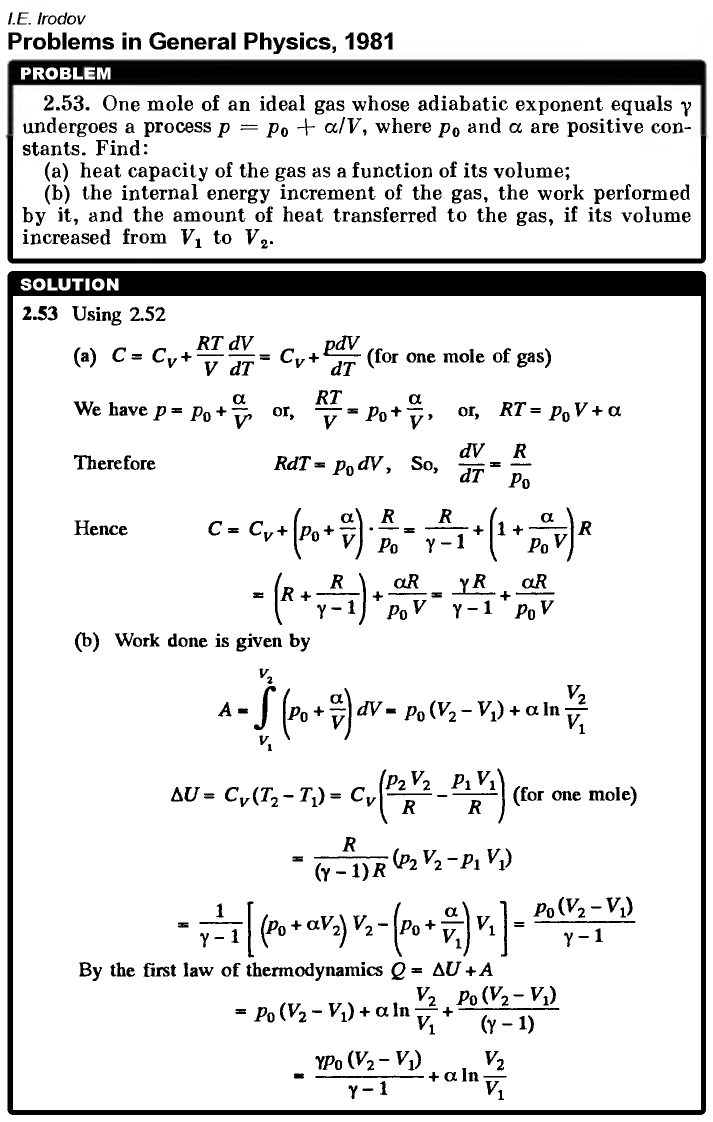
One mole of an ideal gas whose adiabatic exponent equals γ undergoes a process p = p0 + α/V, where p0 and α are positive constants. Find: (a) heat capacity of the gas as a function of its volume; (b) the internal energy increment of the gas, the work performed by it, and the amount of heat transferred to the gas, if its volume increased from V1 to V2.  |
| New search. (Also 5349 free access solutions) |