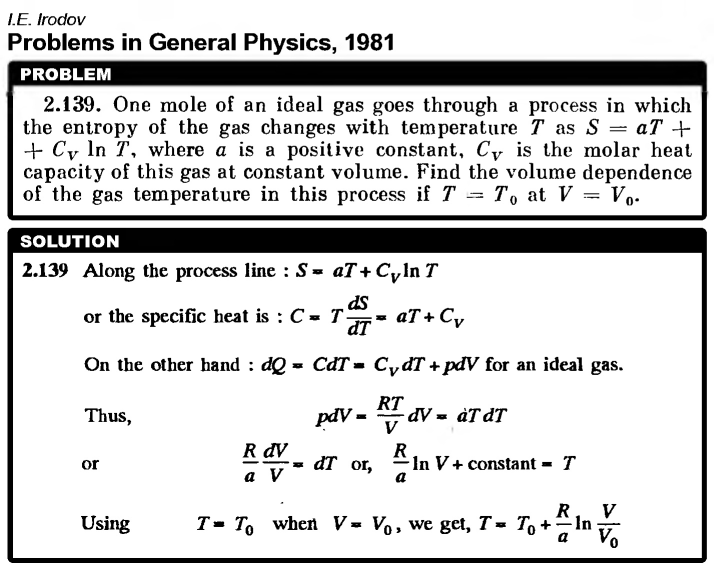
One mole of an ideal gas goes through a process in which the entropy of the gas changes with temperature T as S = aT + Cv ln T, where a is a positive constant, Cv is the molar heat capacity of this gas at constant volume. Find the volume dependence of the gas temperature in this process if T = T0 at V = V0.  |
| New search. (Also 5349 free access solutions) |