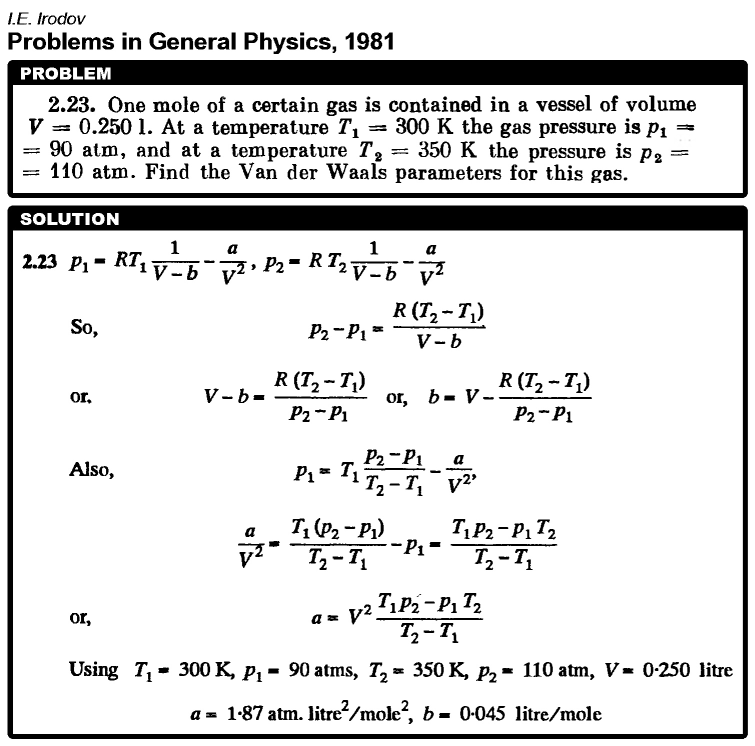
One mole of a certain gas is contained in a vessel of volume V = 0.250 l. At a temperature T1 = 300 K the gas pressure is p1 = 90 atm, and at a temperature T2 = 350 K the pressure is p2 = 110 atm. Find the Van der Waals parameters for this gas.  |
| New search. (Also 5349 free access solutions) |