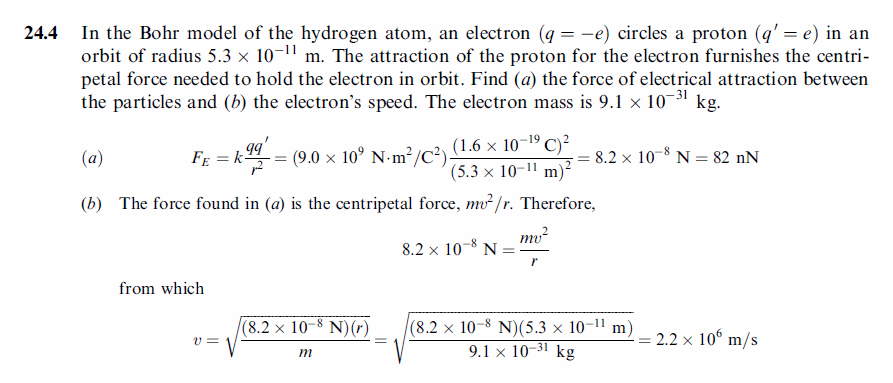
In the Bohr model of the hydrogen atom, an electron (q = —e) circles a proton (q' = e) in an orbit of radius 5.3 X 10^(—1)1 m. The attraction of the proton for the electron furnishes the centripetal force needed to hold the electron in orbit. Find (a) the force of electrical attraction between the particles and (b) the electron’s speed. The electron mass is 9.1 X 10^(—31) kg. |
| New search. (Also 5349 free access solutions) |