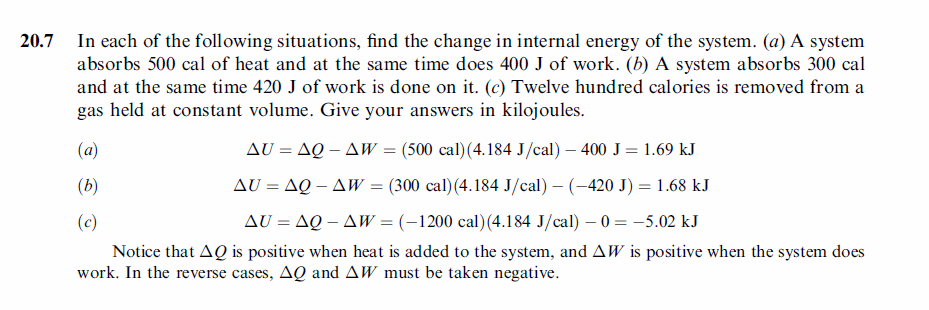
In each of the following situations, find the change in internal energy of the system. (a) A system absorbs 500 cal of heat and at the same time does 400 J of work. (b) A system absorbs 300 cal and at the same time 420 J of work is done on it. (c) Twelve hundred calories is removed from a gas held at constant volume. Give your answers in kilojoules. |
| New search. (Also 5349 free access solutions) |