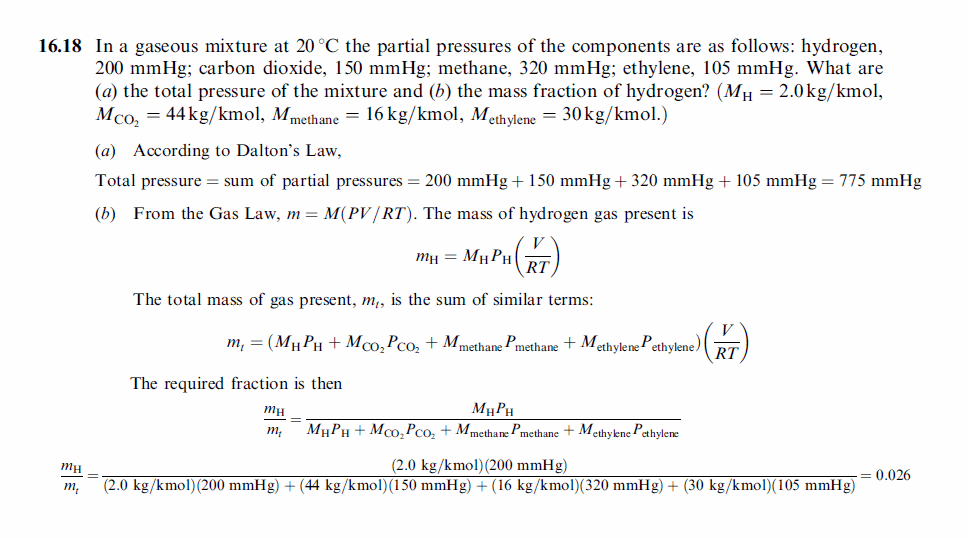
In a gaseous mixture at 20 °C the partial pressures of the components are as follows: hydrogen, 200 mmHg; carbon dioxide, 150 mmHg; methane, 320 mmHg; ethylene, 105 mmHg. What are (a) the total pressure of the mixture and (b) the mass fraction of hydrogen? (MH = 2.0 kg/kmol, MCO2 = 44kg/kmol, Mmethane = 16kg/kmol, Methylene = 30kg/kmol.) |
| New search. (Also 5349 free access solutions) |