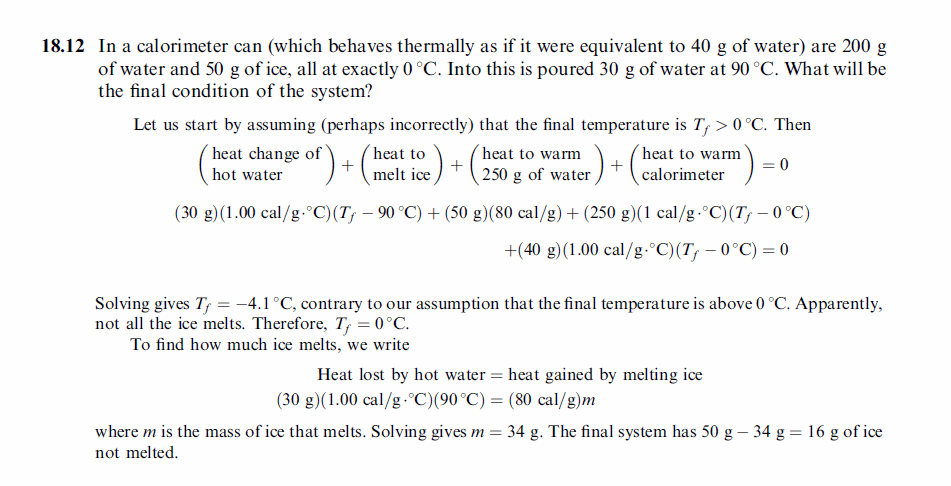
In a calorimeter can (which behaves thermally as if it were equivalent to 40 g of water) are 200 g of water and 50 g of ice, all at exactly 0 °C. Into this is poured 30 g of water at 90 °C. What will be the final condition of the system? |
| New search. (Also 5349 free access solutions) |