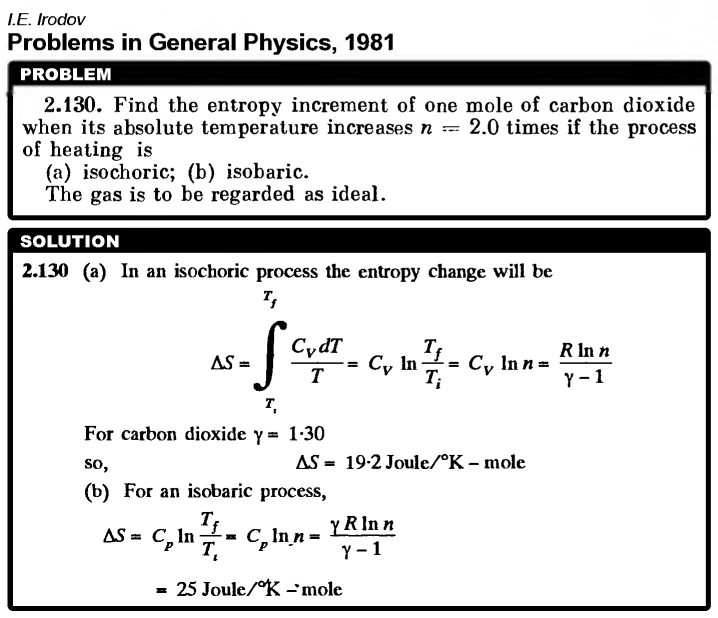
Find the entropy increment of one mole of carbon dioxide when its absolute temperature increases n = 2.0 times if the process of heating is (a) isochoric; (b) isobaric. The gas is to be regarded as ideal. |
| New search. (Also 5349 free access solutions) |