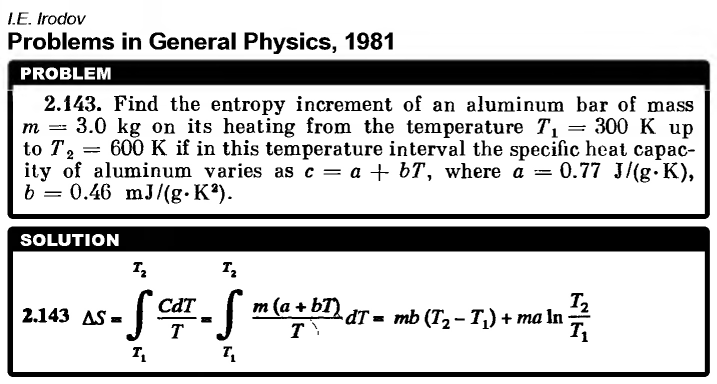
Find the entropy increment of an aluminum bar of mass m = 3.0 kg on its heating from the temperature T1 = 300 K up to T2 = 600 K if in this temperature interval the specific heat capacity of aluminum varies as c = a + bT, where a = 0.77 J/(g*K), b = 0.46 mJ/(g*K2).  |
| New search. (Also 5349 free access solutions) |