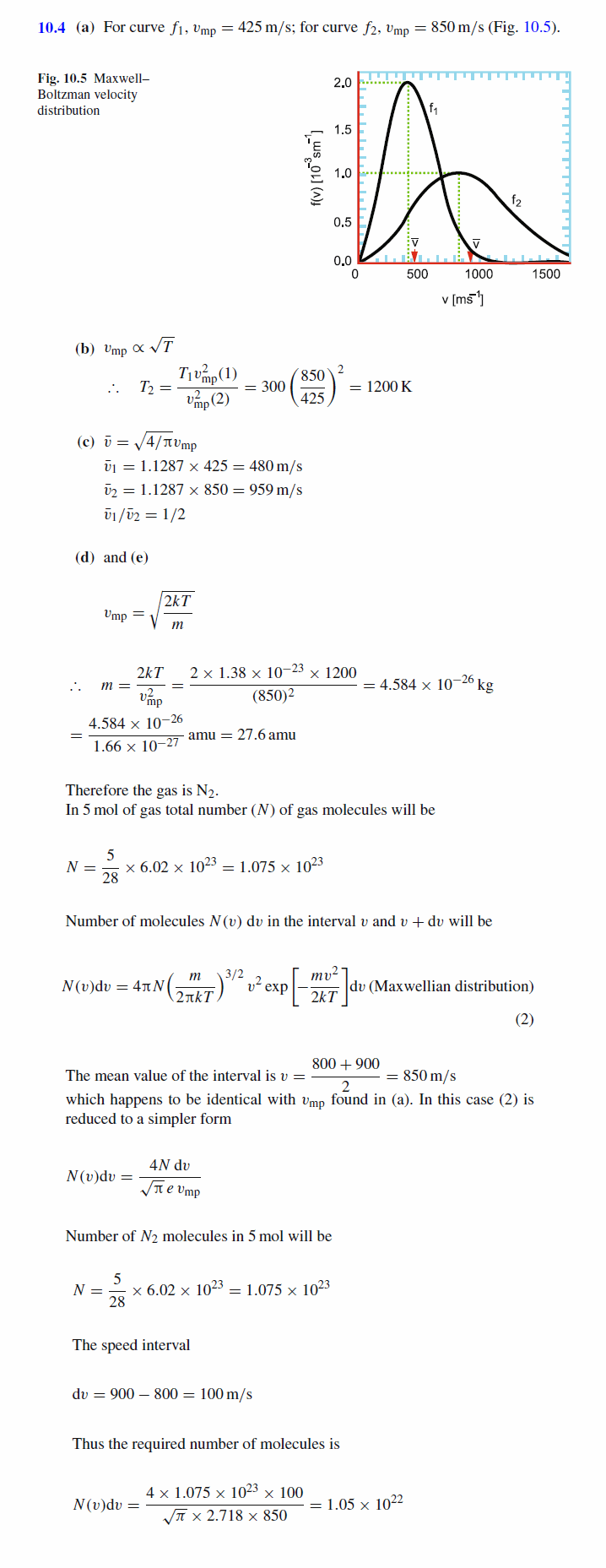
Figure 10.1 shows the Maxwell-Boltzmann velocity distribution functions of a gas for two different temperatures, of which first (curve f1 ) is for T1 = 300 K. (a) Read the approximate value for the most probable speed of the molecules from the diagram for each of the two cases. (b) What is the temperature, T2 , when the velocity distribution is given by f2 ? (c) Indicate the average speeds in the diagram for each of the two temperatures and give the ratio between the two average speeds. (d) The gas consists of 5 mol of molecules. If the molecular velocity distribution is given by f2 , estimate the number of those molecules in the gas which have a speed between vA = 800 m/s and vB = 900 m/s.
Solution:
|

