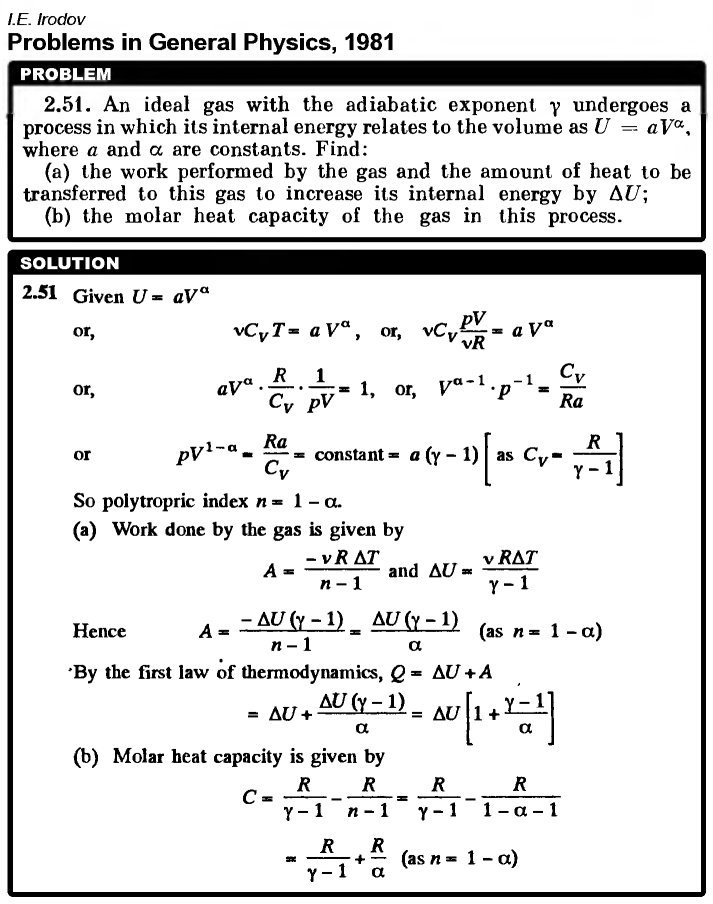
An ideal gas with the adiabatic exponent γ undergoes a process in which its internal energy relates to the volume as U = aVα, where a and α are constants. Find: (a) the work performed by the gas and the amount of heat to be transferred to this gas to increase its internal energy by ΔU; (b) the molar heat capacity of the gas in this process.  |
| New search. (Also 5349 free access solutions) |