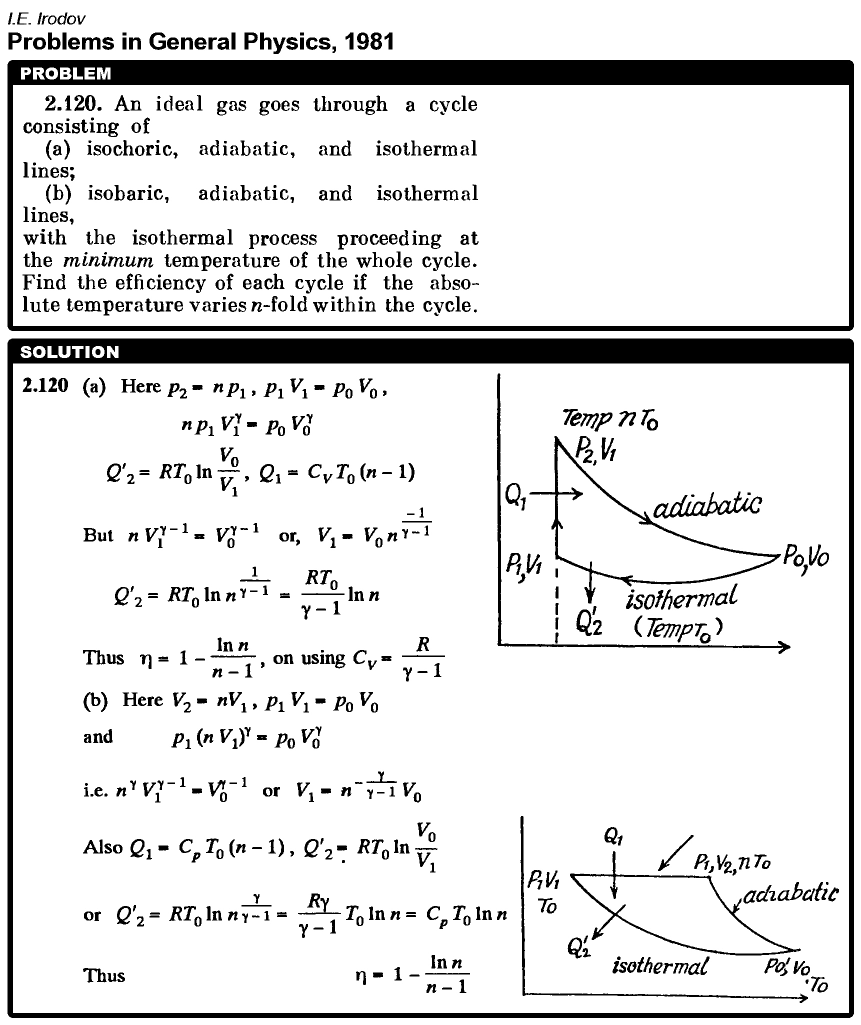
An ideal gas goes through a cycle consisting of (a) isochoric, adiabatic, and isothermal lines; (b) isobaric, adiabatic, and isothermal lines, with the isothermal process proceeding at the minimum temperature of the whole cycle. Find the efficiency of each cycle if the absolute temperature varies n-fold within the cycle.  |
| New search. (Also 5349 free access solutions) |