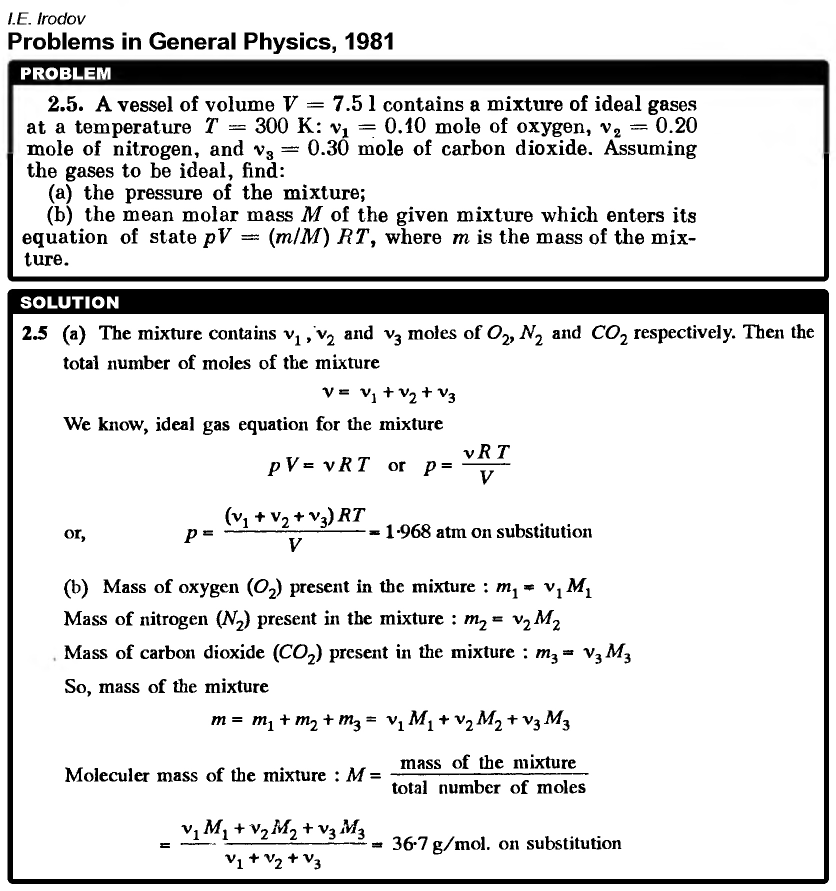
A vessel of volume V = 7.5 1 contains a mixture of ideal gases at a temperature T = 300 K: n1 = 0.10 mole of oxygen, n2 = 0.20 mole of nitrogen, and n3 = 0.30 mole of carbon dioxide. Assuming the gases to be ideal, find: (a) the pressure of the mixture; (b) the mean molar mass M of the given mixture which enters its equation of state pV = (mIM) RT, where m is the mass of the mixture. |
| New search. (Also 5349 free access solutions) |