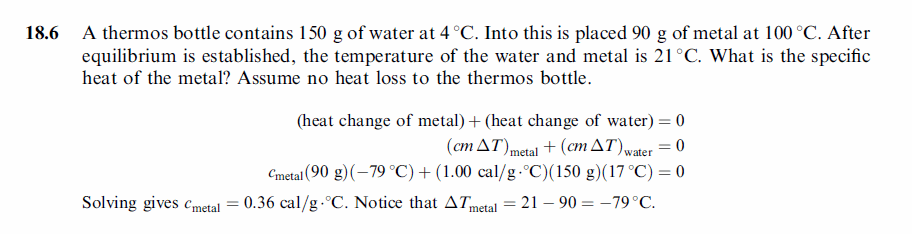
A thermos bottle contains 150 g of water at 4 °C. Into this is placed 90 g of metal at 100 °C. After equilibrium is established, the temperature of the water and metal is 21 °C. What is the specific heat of the metal? Assume no heat loss to the thermos bottle. |
| New search. (Also 5349 free access solutions) |