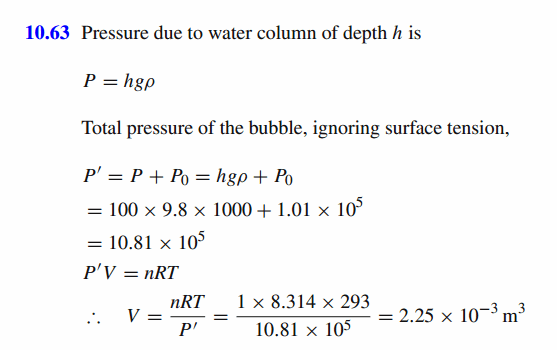
A mole of gaseous molecules in a bubble obeys the ideal gas law. What is the volume of the bubble at a 100 m depth of water if the temperature is 293 K, the atmospheric pressure is 101 kPa, density of water is 1000 kg/m3 and the ideal gas constant is 8.314 J/mol/K. |
| New search. (Also 5349 free access solutions) |