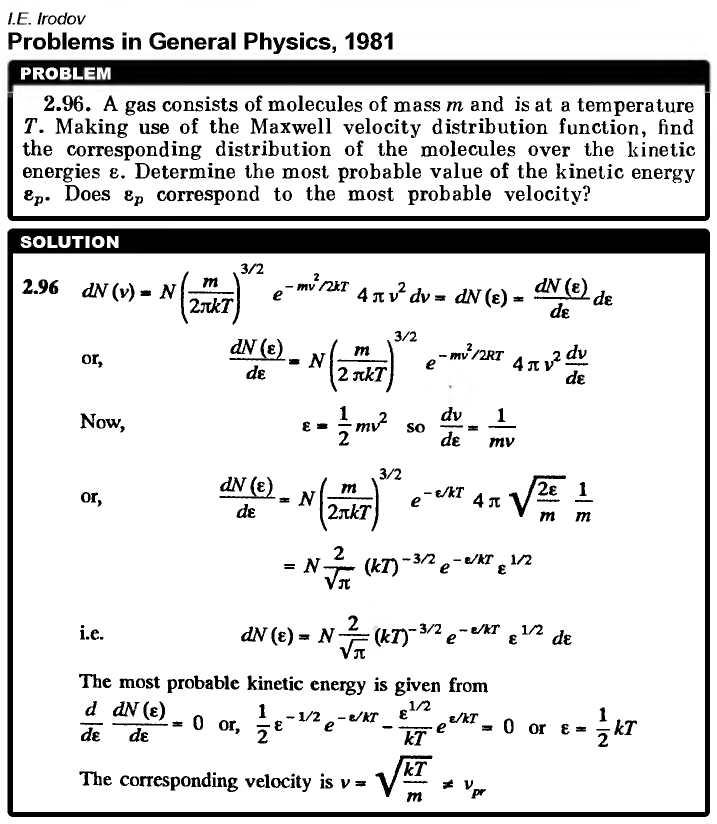
A gas consists of molecules of mass m and is at a temperature T. Making use of the Maxwell velocity distribution function, find the corresponding distribution of the molecules over the kinetic energies c. Determine the most probable value of the kinetic energy ep. Does ep correspond to the most probable velocity? |
| New search. (Also 5349 free access solutions) |