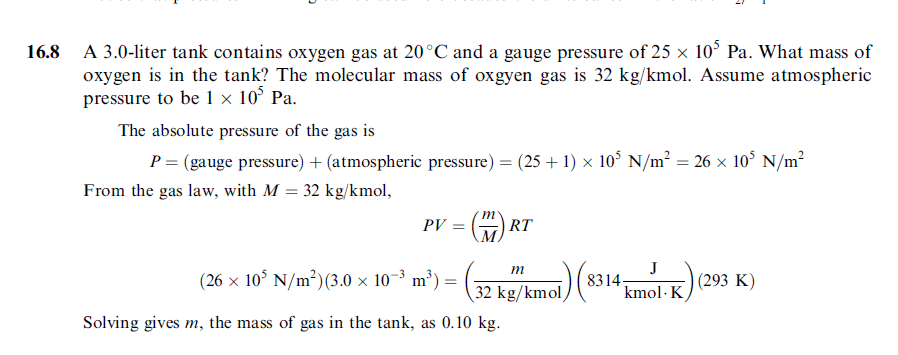
A 3.0-liter tank contains oxygen gas at 20 °C and a gauge pressure of 25 x 10^5 Pa. What mass of oxygen is in the tank? The molecular mass of oxgyen gas is 32 kg/kmol. Assume atmospheric pressure to be 1 x 10^5 Pa.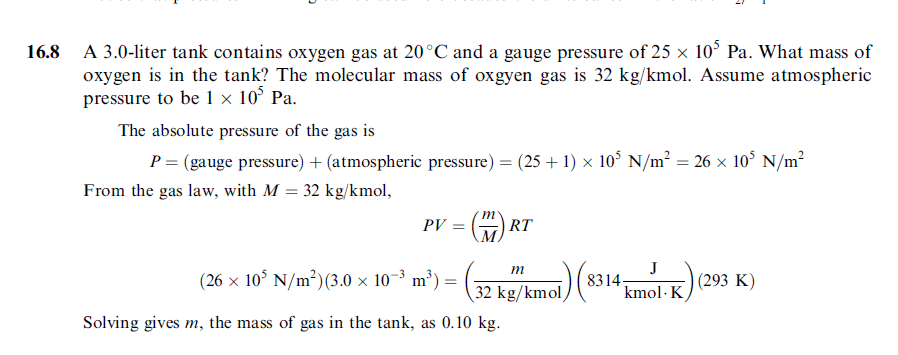 |
| New search. (Also 5349 free access solutions) |