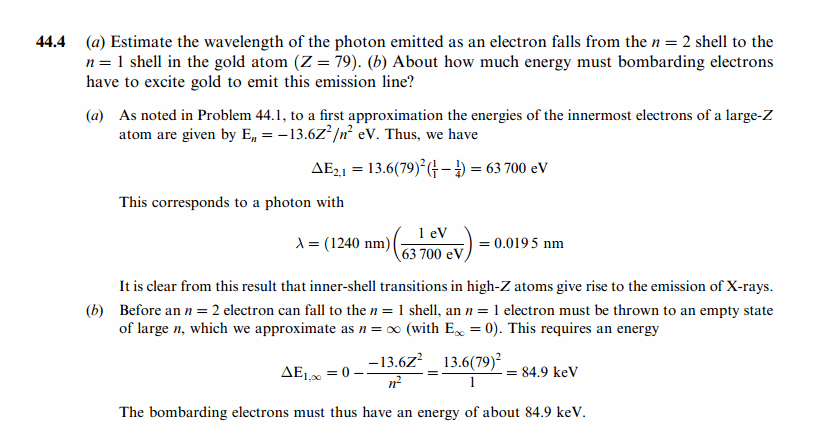
(a) Estimate the wavelength of the photon emitted as an electron falls from the n = 2 shell to the n = 1 shell in the gold atom (Z = 79). (b) About how much energy must bombarding electrons have to excite gold to emit this emission line? |
| New search. (Also 5349 free access solutions) |