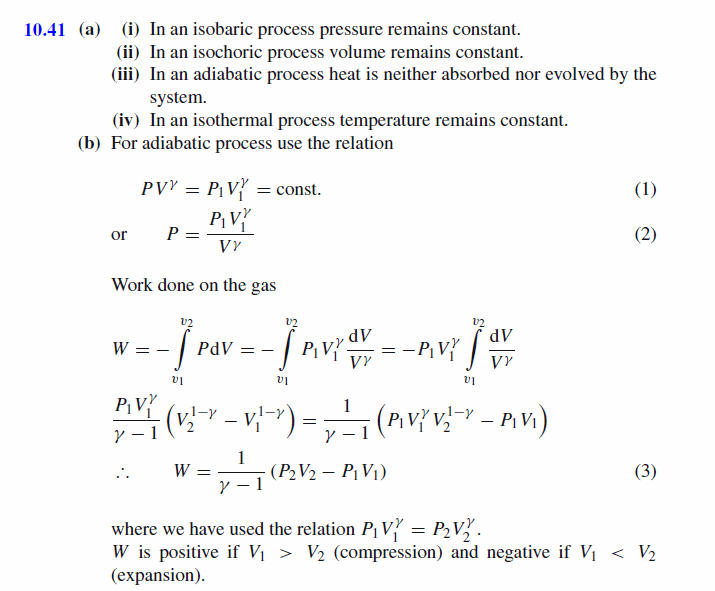
(a) Define (i) an isobaric process (ii) an isochoric process (iii) an adiabatic process (iv) an isothermal process (b) Show that the work done on a gas during an adiabatic compression from initial conditions ( P1 , V1 ) to final conditions ( P2 , V2 ) is given by the equation W = 1/(y-1)(p2V2-p1V1) |
| New search. (Also 5349 free access solutions) |