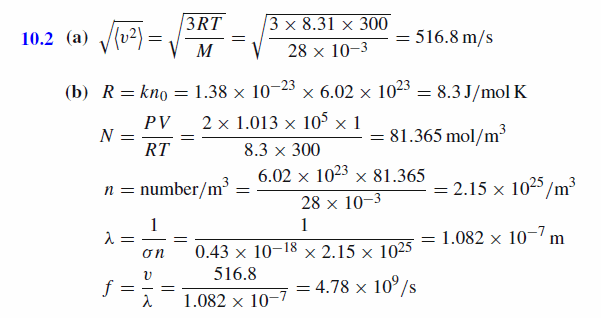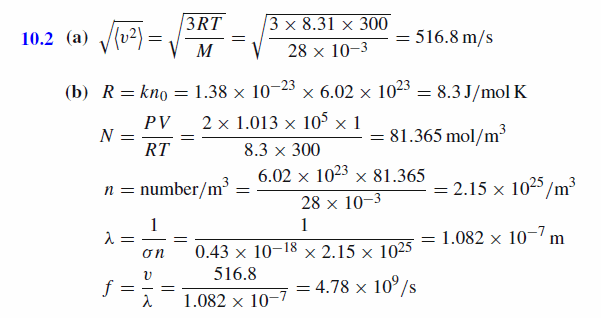(a) Consider a gas that has a molecular weight of 28 and a temperature of 27°C. What is the rms speed of molecule of the gas if it has a Maxwellian velocity distribution? The ideal gas constant is 8.31 J/mol K. (b) What is the mean free path of a molecule if the pressure is 2 atm (1 atm = 101.3 kPa), the temperature is 27°C, and the cross-section is 0.43 nm2 ? Using the average velocity from part (a) calculate the collision frequency for the molecule. Boltzmann's constant is 1.38x10^(-23) J/K.
Solution:
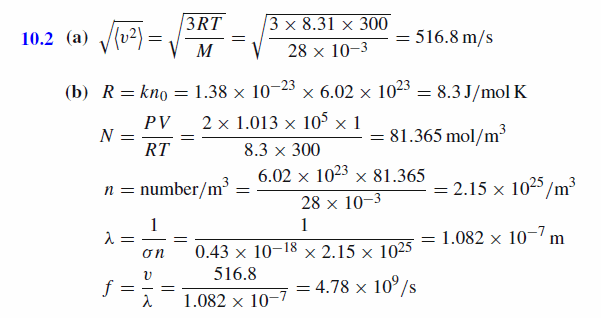
|