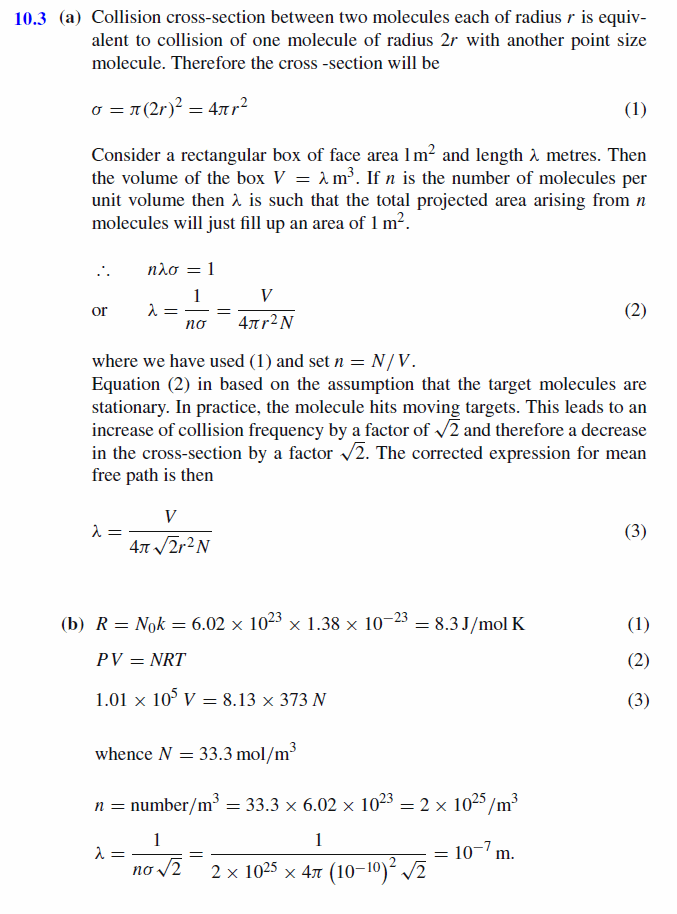
(a) By considering a volume, V , of ideal gas, containing N spherical molecules with radius r , show that the mean free path of the molecules can be defined by the equation L = V/4pi sqrt(2)r^2N (b) Hence, or otherwise, calculate the mean free path of air at 100°C and 1.01x10^5 Pa. Assume that the molecules of air are spheres of radius r = 2.0x10^(-10) m. (k = 1.38x10^(-23) J/K) |
| New search. (Also 5349 free access solutions) |